Thursday, February 22, 2018
Mechanism of urine formation
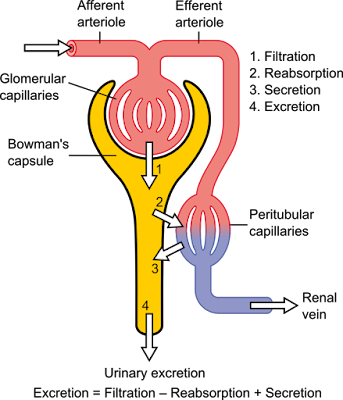
Urine formation and the adjustment of blood composition involve three major processes: glomerular filtration by the glomeruli, tubular reabsorption and tubular secretion in the renal tubules. In addition, the collecting ducts work in concert with the nephrons to make concentrated or dilute urine.
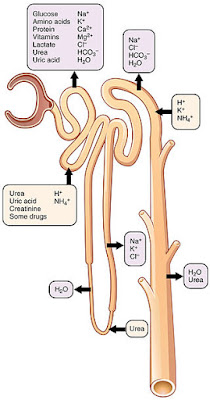
How do the kidneys "clean" the blood? Conceptually, it's really very simple. The kidneys "dump" (by glomerular filtration) (1) cell- and protein-free blood into a separate "container" (the renal tubules and collecting ducts). From this container, the kidneys reclaimed (by tubular reabsorption) (2) everything that the body need to keep. This is almost everything-all of the glucose and amino acid, and some 99% of the water, salt and other components. Anything that is not reabsorbed becomes urine. In addition, some things are selectively added to the container (by tubular secretion) (3)Fine-tuning the bodys chemical balance,
The volume of blood proceed by the kidneys each day is enormous. Of the approximately 1200 ml of blood that passes through the glomeruli each minute, some 650 ml is plasma, and about one-fifth of this (120-125 ml) is forced into the renal tubules. This is equivalent to filtering out your entire plasma volume more than 60 times each day. Considering the magnitude of their task, it is not surprising that the kidneys (which account for only 1% of body weight) consume 20-25% of all oxygen used by the body at rest.
Filtrate and urine are quite different. Filtrate contains everything found in blood plasma except proteins. Urine contains mostly metabolic wastes and unneeded substances. The kidneys process about 180 L of blood-derived fluid daily. Of this amount, less than 1% (1,5L) typically leaves the body as urine, the rest returns to the circulation.
Juxta glomerular apparatus
JUXTA GLOMERULAR APPARATUS
Each nephron has a region called a juxtaglomerular apparatus, where the most distal portion of the ascending limb of the loop of Henle lies against the afferent arteriole feeding the glomerulus (and sometimes the efferent arteriole). Both the ascending limb and the afferent arteriole are modified at the point of contact.
The juxtaglomerular apparatus includes two cell populations that play important roles in regulating the rate of filtrate formation and systemic blood pressure. In the arterioles walls are the granular cells, also called juxtaglomerular cells, which are enlarged, smooth muscle cells with prominent secretory granules containing renin. Granular cells act as mechanoreceptors that sense the blood pressure in the afferent arteriole. The macula densa is a group of tall, closely packed cells of the ascending limb of the loop of Henle that lies adjacent to the granular cells. The macula densa cells are chemoreceptors that respond to changes in the NaCl content of the filtrate. A third population of cells, the extraglomerular mesangial cells, is also part of the juxtaglomerular apparatus. These cells are interconnected by gap junctions and may pass signals between macula densa and granular cells.
THE FILTRATION MEMBRANE
The filtration membrane lies between the blood and the interior of the glomerular capsule. It is a porous membrane that allows free passage of water and solutes smaller than plasma proteins.
The filtration membrane layers are:
- The fenestrated endothelium of the glomerular capillaries
- The visceral membrane of the glomerular capsule, made of podocytes which have filtration slits between their foot processes
- The basement membrane composed of the fused basal laminae of the two other layers
Almost all macromolecules that do manage to make it through the basement membrane are prevented from traveling further by thin membranes (slit diaphragms) that extend across the filtration slits. Macromolecules that get "hung up" in the filtration membrane are engulfed and degraded by mesangial cells can also contract, changing the total surface area of the capillaries available for filtration.
Wednesday, February 21, 2018
Nephron capillary beds
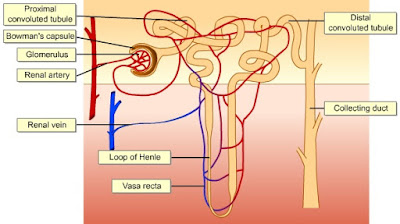
The renal tubule of every nephron is closely associated with two capillary beds: the glomerulus and the peritubular capillaries. The glomerulus, in which the capillaries run in parallel, is specialized for filtration. It differs from all other capillary beds in the body in that it is both fed and drained by arterioles-the afferent arteriole and the efferent arterioles, respectively.
The afferent arterioles arise from the cortical radiate arteries that run through the renal cortex. The blood pressure in the glomerulus is extraordinarily high for a capillary bed because arterioles are high-resistance vessels and the afferent-arteriole has a larger diameter than the efferent. This high blood pressure easily forces fluid and solutes out of the blood into the glomerular capsule. Most of the filtrating filtrate (99%) is reabsorbed by the renal tubule cells and returned to the blood in the peritubular capillary beds.
The peritubular capillaries arise from the efferen arterioles draining the glomeruli. These capillaries cling closely to adjacent renal tubules and empty into nearby venules. They are low-pressure, porous capillaries that readily absorb solutes and water from the tubule cells as these substances are reclaimed from the filtrate.
The efferent arterioles serving the juxtamedullary nephrons tend not to break up into meandering peritubular capillaries. Instead they form bundles of long straight vessels called vasa recta that extend deep into the medulla paralelling the longest loops of Henle. The thin -walled vasa recta play an important role in forming concentrated urine.
In summary, the microvasculature of the nephrons consists of two capillary beds separated by intervening efferent arterioles. The first capillary bed (glomerulus) produce the filtrate. The second (peritubular capillaries) reclaims most of that filtrate.
Blood flowing through the renal circulation encounters high resistance, first in the afferent and then in the efferent arterioles. As a result, renal blood pressure declines from approximately 95mmHg in the renal arteries to 9 mmHg or less in the renal veins. The resistance of the afferent arterioles protects the glomeruli from large fluctuations in systemic blood pressure. Resistance in the efferent arterioles reinforces the high glomerular pressure and reduces the hydrostatic pressure in the peritubular capillaries.
Monday, February 19, 2018
Nephrons
Nephrons are the structural and functional units of the kidneys. Each kidney contains over 1 million of these tiny blood-processing units, which carry out the process that form urine. In addition, there are thousands of collecting ducts, each of which collects fluid from several nephrons and conveys it to the renal pelvis.
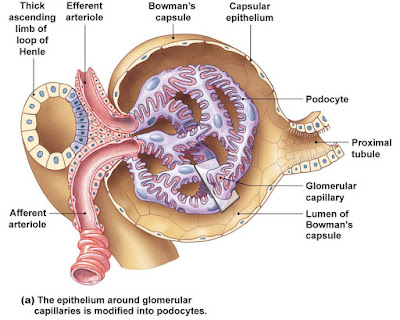
Each nephron consist of a glomerulus, which is a tuft of capillaries, and a renal tubule. The renal tubule has a cup shaped-end, the glomerular capsule (or Bowman's capsule), which is blind and completely surrounds the glomerulus, much as a well-worn baseball glove encloses a ball. Collectively, the glomerular capsule and the enclosed glomerulus are called the renal corpuscle.
The endothelium of the glomerular capillaries is fenestrated (penetrated by many pores), which makes them exceptionally porous. This allows large amounts of solute-rich, virtually protein-free fluid to pass from the blood into the glomerular capsule. This plasma-derived fluid or filtrate is the raw material that the renal tubules process to form urine.
The external parietal layer of the glomerular capsule is simple squamous epithelium. This layer simply contributes to the capsule structure and plays no part in forming filtrate.
The visceral layer, which clings to the glomerular capillaries, consist of highly modified, branching epithelial cells called podocyte. The octopus-like podocytes terminate in foot processes, which intertwine as they cling to the basement membrane of the glomerulus. The clefts or opening between the foot processes are called filtration slits. Through these slits, filtrate enters the capsular space inside the glomerular capsule.
The remainder of the renal tubule is about 3 cm long and has three major parts. It leaves the glomerular capsule as the elaborately coiled proximal convulated tubule, make a hairpin loop called the loop of Henle (also called the nephron loop or Henle's loop), and then winds and twists again as the distal convoluted tubule before emptying into a collecting duct. The terms proximal and distal indicate relationship of the convoluted tubules to the renal corpuscle-filtrate from the renal corpuscle passes through the proximal convulated tubule first and then the distal convulated tubule, which is thus "further away" from the renal corpuscle. The meandering nature of the renal tubule increases its length and enhances its filtrate processing capabilities.
The collecting ducts, each of which receives from may nephrons, run through the medullary pyramids and give them their striped appearance. As the collecting ducts approach the renal pelvis, they fuse together and deliver urine into the minor calyces via papillae of the pyramids.
The U-shaped loop of Henle has decending and ascending limbs. The proximal part of the decending limb is continuous with the proximal tubule and its cells are similar. The rest decending limb, called the thin segment, is a simple squamous epithelium freely permeable to water. The epithelium becomes cuboidal or even low columnar in the ascending part of the loop of Henle, which therefore becomes the thick segment. In some nephrons, the thin segment is found only in the descending limb. In others, it extends into the ascending limb as well.
Nephrons are generally divided into two major groups. Cortical nephrons represent 85% of the nephrons in the kidneys. Except for small parts of their loops of Henle that dip into the outer medulla, they are located entirely in the cortex. The remaining juxtamedullary nephrons originate close to the cortex-medulla junction, and they play an important role in the kidneys ability to produce concentrated urine. Their loops of Henle deeply invade the medulla, and their thin segments are much more extensive than those those of cortical nephrons.
Blood and nerve supply for the kidneys
The kidneys continuosly cleanse the blood and adjust its composition, so it is not surprising that they have a rich blood supply. under normal resting conditions, the large renal arteries deliver one-fourth of the total cardiac output (about 1200 ml) to the kidneys each minute.
The renal arteries issue at right angles from the abdominal aorta and the right renal artery is longer than the left because the aorta lies to the left of the midline. As each renal artery approaches a kidney, it divides into five segmental arteries. Within the renal sinus, each segmental artery branches further to form several interlobar arteries.
At the medulla-cortex junctions, the interlobar arteries branch into the arcuate arteries that arc over the bases of the medullary pyramids. Small cortical radiate arteries radiate outward from the arcuate arteries to supply the cortical tissue. More than 90% of the blood entering the kidney perfuses the renal cortex.
Afferent arterioles branching from the cortical radiate arteries begin a complex arrangement of microscopic blood vessels. These vessels are key elements of kidney function, and we will examine them in detailed later when we describe the nephrons.
Veins pretty much trace the pathway of the arterial supply in reverse. Blood leaving the renal cortex drains sequentially into the cortical radiate, arcuate, interlobar and finally renal veins (there are no segmental veins). The renal veins issue from the kidneys and empty into the inferior vena cava. Because the inferior vena cava lies to the right of the vertebral column, the left renal vein is about twice as long as the right.
The renal plexus, a variable network of autonomic nerve fibers and ganglia, provides the nerve supply of the kidney and its ureter. An offshoot of the celiac plexus, the renal plexus is largely supplied by symphatetic fibers from the most inferior thoracic and first lumbar sphlanchnic nerves, which course along with the renal artery to reach the kidney. These sympathetic vasomotor fibers regulate renal blood flow by adjusting the diameter of renal arterioles and also influence the urine-forming role of the nephrons.
Kidneys anatomy
Location and external anatomy
The bean-shaped kidneys lie in retroperitoneal position (between the dorsal body wall and the parietal peritoneum) in the superior lumbar region. Extending approximately from T12 to L3, the kidneys receive some protection from the lower part of the rib cage. The right kidney is crowded by the liver and lies slightly lower than the left. An adult kidney has a mass about 150g (5 ounces) and its average dimensions are 12 cm long, 6cm wide and 3 cm thick - about the size of a large bar of soap. The lateral surface is convex. The medial surface is concave and has a vertical cleft called the renal hilum that leads into an internal space within the kidney called the renal sinus. The ureter, renal blood vessels, lymphatics and nerves all join each kidney at the hilum and occupy the sinus. Atop each kidney is an adrenal (or suprarenal) gland, an endocrine gland that is functionally unrelated to the kidney.
Three layers of supportive tissue surround each kidney:
- The renal fascia, an outer layer of dense fibrous connective tissue that anchors the kidney and the adrenal gland to surrounding structures
- The perineal fat capsule, a fatty mass that surrounds the kidney and cushions it against blows
- The fibrous capsule, a transparent capsule that prevents infections in surrounding regions from spreading to the kidney
Internal Anatomy
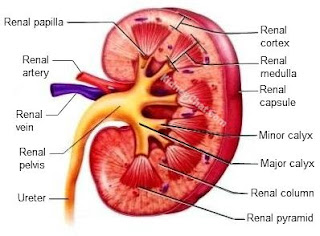
A frontal section through a kidney reveals three distinct regions: cortex, medulla and pelvis. The most superficial region, the renal cortex, is light in color and has a granular appearance. Deep to the cortex is the darker, reddish-brown renal medulla, which exhibits cone-shaped tissue masses called medullary or renal pyramids. The broad base of each pyramids faces toward the cortex, and its apex, or papilla, points internally. The pyramids appear striped because they are formed almost entirely of parallel bundles of microscopic urine-collecting tubules and capillaries. The renal columns, inward extensions of cortical tissue, separate the pyramids. Each pyramid and its surrounding cortical tissue constitutes one of approximately eight lobes of a kidney.
The renal pelvis, a funnel-shaped tube, is continuos with the ureter leaving the hilum. Branching extensions of the pelvis form two or three major calyces. Each one subdivides to form several minor calyces, cup-shaped areas that enclose the papillae.
The calyces collect urine, which drains continuosly from the papillae, and empty it into the renal pelvis. The urine then flows through the renal pelvis and into ureter, which moves it to the bladder to be stored. The walls of the calyces, pelvis and ureter contain smooth muscles that contracts rhytmically to propel urine along its course by peristalsis.
Sunday, February 18, 2018
The Urinary System
Every day the kidneys filter nearly 200 liters of fluid from the blood stream, allowing toxins, metabolic wastes and excess ions to leave the body in urine while returning needed substances to blood. Much like a water purification plant that keep a city's water drinkable and disposes of its wastes, the kidneys are usually unappreciated until they malfunction and body fluids become contaminated. Although the lungs and skin also participate in excretion, the kidneys are the major excretory organs.
As the kidneys perform these excretory functions, they also act as essential regulators of the volume and chemical makeup of the blood, maintaining the proper balance between water and salts and between acids and bases. Frankly, this would be tricky work for a chemical engineer, but the kidneys do it effeciently most of the time.
Besides the urine-forming kidneys, the urinary system includes the urinary bladder, a temporary storage reservoir for urine, plus three tubelike organs-the paired ureters and the urethra, all three of which furnish transportation channels for urine.
Other renal functions include:
- Gluconeogenesis during prolonged fasting
- Producing the hormons renin and erythropoetin. Renin acts as an enzyme to help regulate blood pressure and kidney function. Erythropoetin stimulates red blood cell production
- Metabolizing vitamin D to its active form
Thursday, February 15, 2018
Wednesday, February 14, 2018
Renal disorders in the newborn
Prenatal diagnosis of renal disease is usually by fetal ultrasonograms that detect signs of obstructive uropathy. Fetal hydrops may occur with congenital nephrotic syndrome. Oligohidramnios occurs with severe urinary tract obstruction or renal agenesis, which is associated with pulmonary hypoplasia (Potter's syndromes).
Clinical manifestations of renal disease vary with the type and severity of abnormality. Certain findings are indicative or suggestive of renal disease :
- Potter's syndrome
- Dysmorphic features
- Lateral abdominal mass
- Ascites
- Suprapubic mass
- Abdominal wall defects
- Failure to palpate kidney
- Hypertension
- Anuria or oliguria
The presence of any of the above signs should alert one to the possibility of renal dysfunction and raise the possibility of further diagnostic work-up including, in addition to careful measurements of intake and urine output, serum creatinine, blood urea nitrogen, electrolytes and abdominal ultrasound.
Tuesday, February 13, 2018
Dysplastic kidney disease
Dysplastic kidney disease is common, occuring as unilateral disease in 1 in 1000 births and bilaterally in 1 in 5000. It is the common cause of an abdominal mass in the newborn. The condition typically is diagnosed on prenatal ultrasonography. The kidney is highly echogenic or "bright", with multiple large, thin-walled cysts that typically are seen by 20 weeks gestation, in dystiction from polycystic disease. These cysts characteristically separate and distributed randomly througout the renal parenchyma. Grossly, the kidneys are dysplastic, with little normal architecture evident, and the multiple large cysts are easily throughout the entire organ. Most often, an affected kidney is significantly enlarged, and this large irregular mass may be easily palpated in the newborn. Both the cysts and overall enlargement may become more pronounced as gestation proceeds, but many dysplastic kidneys involute, even during the prenatal period. Unlike cases of obstruction of the lower collecting systems, it can be difficult to identify the renal pelvis or ureter on ultrasonography.
Bilateral disease frequently is severe, and the prognosis typically poor, with progression to severe oligohydramnios and pulmonary hypoplasia, resulting in Potter sequence, and a low likelihood of survival after birth. This outcome usually associated with findings of either marked renal enlargement or involution during late pregnancy. Some cases of bilateral disease are less severe and survival is possible. Dialysis is necessary for chronic renal failure in affected children, and their require specialized care for nutrition. As the children grow, renal transplantation becomes a possibility.
Unilateral disease usually presents with no specific signs or symptoms in the neonate, other than the presence of a large abdominal mass, which rarely can cause mechanical problems. If it is, of course, diagnosed typically on prenatal ultrasonography
Following a prenatal diagnosis, postnatal ultrasonography should be undertaken to rule out associated urinary tract anomalies and to confirm the original diagnosis. Many practitioners recommend waiting for at least 1 week after birth to perform this study to avoid false-negative results due to normal oliguria, but others have found that studies performed as early as 48 hours after birth are reliable, especially if repeated at about age 6 weeks.
The major risk is the development of chronic renal failure, which develops in 12% to 50% of patients who have apparently normal contralateral kidneys. Serum creatinine is monitored routinely to identify developing renal failure. Hypertension is uncommon occurence, and blood pressure monitoring is recomended only as part of regular care.
Polycystic kidney disease
Polycystic kidney disease occurs as two distinc genetic disorders, autosomal dominant and autosomal recessive forms. The incidence of polycystic kidney disease is 1 in 1000 live births, making it the most common of the inherited disorders. Autosomal dominant polycystic kidney disease, in which bilateral renal cysts form and progress, rarely presents in neonate. When it does, the presentation is similar to that of the recessive form, with a broad range of severity from asymptomatic through severe renal dysfunction, along with variable degrees of hypertension. As a result, the parents of any baby who has polycystic kidney disease should themselves undergo ultrasonographic examinations to rule out the small possibility of the disease being the autosomal dominant form. any patients have no renal symptoms through childhood, but in the long term, more than 50% deteriorate progressively to end-stage disease, often over decades.
The clinical presentation includes polycystic kidneys, congenital hepatic fibrosis, and some degree of biliary dysgenesis, although hepatic symptoms are uncommon in neonatus. The renal manifestations vary in severity but generally include large kidneys that appear echogenic on prenatal ultrasonography, with initially normal amniotic fluid volume. Characteristically, the kidney filled with multiple small cysts that are not easily visible on early ultrasonography and only appear as gestation proceeds, although the kidneys are large and echogenic. The gross appearance of the kidney mirrors of the ultrasonographic findings, with numerous small cysts throughout the parenchyma.
In more severe cases of autosomal recessive polycystic kidney disease, the amniotic volume declines as gestation progresses, and in many cases, oligohydramnios is present by late in the second trimester. These more severely affected fetuses may develop the Potter phenotype, with pulmonary hypoplasia and a small chest, beaked and flattened nose, and deformations of the extrimities and spine. This phenotype is most likely to develop when severe oligohydramnios is present by mid-gestation. In these instances, the degree of lung hypoplasia may be critical, making extrauterine survival imposible.
Among a recent large cohort of patients for whom prenatal diagnosis is available, the early mortality was slightly more then 25%, primarly as a result of respiratory failure and sepsis.A total of 41% of patients required mechanical ventilation after birth, and almost 12% of the survivors developed chronic lung disease. Longer term, 42% developed chronic renal insufficiency, and more than 25% of patients manifested slowed or delayed growth in infancy and early childhood, related to poor renal and pulmonary function. Importantly, the median age of the patients was 5,4 years, demonstrating that survival at least through childhood is possible with this disease.
Renal replacement therapy and prognosis in the infant
Congenital anomalies of the kidneys and urinary tract include a wide range of defects that have their origin during the critical period of organogenesis of urinary system. The conditions includes a full spectrum and array of defects and can include minor conditions, such as mild resolving hydronephrosis, all the way to anuric renal failure due to posterior urethral valves or bilateral renal agenesis. Its etiologies can include many of genes known to be critical in urinary tract development, as well as environtmental factors that can interfere with development at key stages, however, in the majority of cases, the etiology remains unknown. Most cases can be detected and diagnosed prenatally with modern antenatal ultrasonography.
Lower urinary tract obstruction secondary to posterior urethral valves shows a biphasic peak incidence for time to progression to end-stage renal disease, with the first peak being in infancy and the second peak occuring in adolescents. In the most severe cases, the kidneys are so dysplastic and poorly developed that there is severe oliguric kidney failure at the time of birth or very soon afterwards. Many of the seemingly less severe cases still result in highly dysplastic kidneys, which display poor growth over time, eventually culminating in end-stage renal disease.
Infant dialysis is technically challenging and costly, therefore, it would be helpful to have a clear idea of which patients would be at highest risk for early renal failure. Dialysis can be problematic in the newborn and requires strong nephrologic expertise, which may not available at all institution. However, over the last 20 years, renal replacement therapy has improved outcomes in infants and children, and success has been reported.
For children with end-stage renal disease, dialysis is generally considered a bridging step until kidney transplantation is possible. Assesing the odds for successful kidney transplantation, 10 kg is generally agreed to be the smallest size. To support a newborn with end-stage renal disease to the 10 kg mark, intensive nutritional therapy, often with tube feeding, is required. These infants generally have oral aversions and have failure to thrive due to frequent vomiting and an increased catabolism.
Saturday, February 10, 2018
Prenatal diagnosis of congenital anomalies of the kidneys and urinary tract
The widespread use and sensitivity of fetal ultrasonography has resulted in antenatal detection of the majority of renal malformations. Ultrasonographic screening for fetal anomalies can detect renal agenesis, multicystic dysplastic kidney, hydronephrosis, and abnormally shaped bladders from midway through gestation.
Fetal urinary production starts at 9 weeks gestation, therefore, the fetal bladder should be visualized from 13 weeks onward. By 20 weeks.fetal urine produces 90% of amniotic fluid. References curves for renal volume and the amount of amniotic fluid are available. Fetal kidneys can be visualized at 12 weeks, and by 25 weeks, the renal cortex and medulla are distincly demonstrated on ultrasonography. Parameters for expected length (appropriate growth) based on gestational age are also available.
Fetal hydronephrosis is most commonly detected during routine ultrasonography between 18 and 22 weeks gestation. Several systems has been developed to diagnose and grade the severity of hydronephrosis, but there is no consensus on the most appropriate criteria. In general, the likelihood of having a significant renal anomaly correlates with the severity of hydronephrosis. Because of renal immaturity, hydronephrosis detected before 25 weeks warrants repeat scanning. As expected, studies performed in the third semester are the most helpful in predicting the postnatal outcome of congenital anomalies of the kidneys and urinary tract. Thinning of the renal parenchyma and/or cortical cyst may be seen with hydronephrosis. They indicate injury or impaired development of the renal cortex. Increased echogenicity of the renal cortex may indicate anbormalrenal parenchymal development. These findings are associated with poor postnatal renal function when combined with hydronephrosis.
Normally, the fetal ureters are not seen on ultrasonography, so when they are visualized, ureteric or bladder obstruction or vesikooreteral reflux may be indicated. Bilateral involvement increases the risk of significant renal abnormality and of impaired postnatal renal function. The bladder wall is normally thin. If the bladder wal is thick, urethral obstruction such as posterior uretral valves in a male fetus may be present. If the bladder is not seen, consider the diagnosis of bladder extrophy.
Oligohydramnions at or beyond the 20th week of gestation is the most reliable predictor of abnormal fetal renal functions. Because amniotic fluid is predominantly composed of fetal urine, biochemical analysis is useful in further assesing fetal renal function. Sodium and chloride concentration greater than 90 mEq/L (90mmol/L) and urinary osmolality less than 210 mOsm/kg H2O (210 mmol/kg H2O) in the amniotic fluid are indicative of fetal renal tubular impairement and poor renal prognosis. Under these circumstance, more invasive testing such as vesicocentesis (bladder taps) may be undertaken.
Embryonic development of the kidney and urinary tract
Because of the complex nature of renal development, there are multiple opportunities for interference, through mutations in crucial genes or environtmental stresses, affecting various stages in the ontogeny of the kidney, which unfortunately lead to the outcome of a congenital anomalies of the kidneys and urinary tract disorders. In humans, the kidneys develop through a multistep process, progressing from an anterior to posterior tract. To produce mature kidneys, 2 transient phases must be navigated. These are characterized by partial development, followed by regression of primitive kidney, and then a third and final phase that results in a pair of functioning kidneys that serve to filter blood, excrete metabolic waste products, and regulate plasma electrolyte concentration and osmolality.
The process begins with formation of the nephritic duct (day 22 in humans) in the intermediate mesoderm at an anterior position. The duct induces nearby intermediate mesoderm cells to form the primitive tubules of the first kidney, known as the pronephros. In humans, the pronephros quickly degenerates, never forming a functioning renal organ. As the tubules of the pronephros dissapear, the nephritic duct grows caudally, following parallel to the tract of intermediate mesoderm. At a location caudal to the pronephros, the signals of the nephric duct transform the intermediate mesoderm into the next primitive kidney, the mesonephros. Like the pronephros, the mesonephros forms a series of tubules (day 25 in humans), but degenerates after a short time. However, in the case of mesonephros, parts of tubules remain, becoming components of the male reproductive tracts (vas deferens), but do not contribute to the final kidney.
Still further caudal, near the level of the developing hind limb, is the metanephric mesenchyme, which will eventually develop into the final kidneys if the proper signaling cascade is achieved. The metanephric mesenchyme cells produce and secrete glial cell line-derived neurotrophic factor, once the nephric duct reaches the level of the metanephric mesenchyme and is exposed to the chemical gradient of glial cell line-derived neurotrophic factor, it responds by producing a diverticula that homes in on, and invades, the metanephric mesenchyme tissue. This diverticula coming of the nephric duct is known as the ureteric bud, and it reciprocates the chemical communication with the metanephric mesenchyme by secreting a number of transcription factors, including Fgf2 and BMP7, which are necessary to prevent the cells of the metanephric mesenchyme from undergoing apoptosis, as occured with the pronephros and mesonephros. The metanephric mesenchyme is the only tissue that can respond to ureteric bud signaling because of expression in this tissue.
As the ureteric bud invades the metanephric mesenchyme, it will begin a series of branching events influenced by glial cell line-derived neurotrophic factor. Cells from the metanephric mesenchyme that condense onto the tips of the ureteric bud (known as cap mesenchyme) will eventually form the nephron. The proper number of branching events by ureteric bud is critical to achieve the appropriate number of nephrons in the final kidney (with normal variation ranging from 300.000 to 1.000.000).
The branches of the ureteric bud eventually form the collecting ducts, renal pelvis and the ureters. The cap mesenchyme that condensed onto the branches of the ureteric bud go on to become the glomerulus, proximal tubule, loop of Henle and distal tubule. The bladder itself originates from a portion of the cloaca, and the nephric duct empties into the bladder, under the direction of Hox genes. All these events need to be precise in both time and space in order for the kidneys, ureters, and bladder to form properly.
Thursday, February 8, 2018
Congenital diseases of the kidneys part 2
COMMON TYPES OF CONGENITAL ANOMALIES OF THE KIDNEYS AND URINARY TRACT :
- Hydronephrosis
- Renal parenchymal malformation
- Disordered renal migration and collecting system formation
SYNDROMES THAT CAN BE ASSOCIATED WITH VACTERL :
- SINGLE GENE DEFECTS
- Alagile syndrom (JAG1 gene)
- Holt-Oram syndrom (TBX gene)
- Smith-Lemli-Opitz syndrome (DHCR7 gene inborn error in cholesterol synthesis)
2. CHROMOSOMAL ANOMALIES (TRISOMY 18)
Heart defects, microcephaly, cleft palate, rocker bottom feet, growth delay, developmental delay, renal defects (horseshoe kidney, hydronephrosis, duplicated collecting system)
Congenital diseases of the kidneys part 1
Chronic kidney disease is a growing public health problem with a huge economic burden on society. In children, congenital anomalies of the kidneys and urinary tract are the most common cause for chronic kidney dieseases. Normal development of the kidneys and urinary tract progresses through a complex series of events and requires the expression of key transcription factors to occur with precision in the fetus. It is now known that many genetic defects can lead to congenital anomalies of the kidneys and urinary tract. Most of them can be indentified prenatally with antenatal ultrasonography. For infants born with severe renal impairment, transfer to a center specializing in infant dyalisis has been shown to be reasonably good, and survival improves further if kidney transplantation can eventually be achieved.
Congenital anomalies of the kidneys and urinary tract is not a single disease but merely descriptive for a large collection of diverse developmental disorders that arise during the formation of the kidneys, ureters, bladder and urethra in fetal life. They are not unified in the sense of having any single genetic etiology or common pattern of injury but can result from a wide array of genetic and/or environtmental factors that make an impact on the precise orchestration and timing of organogenesis of the kidneys and urinary tract.
Congenital anomalies of the kidneys and urinary tract include structural defects as antenatal hydronephrosis, renal agenesis, renal dysplasia, renal ectopia,ureteropelvic junction obstruction, vesicoureteral reflux, duplicated renal collecting systems, and posterior urethral valves.
Congenital anomalies of the kidneys and urinary tract can be seen with single gen mutations, such as Alagille syndrome, caused from a mutation in the JAG1 gene, and showing defects in the liver, heart, face and kidneys. The kidneys anomalies in the Alagille syndrome can vary in severity but can include renal agenesis, hypoplastic kidneys, , ureteropelvic junction obstructions and vesicoureteral reflux.
Other disorders with a congenital anomalies of the kidneys and urinary tract component will be associated with well recognized syndromes or nonrandom associations, displaying both renal and extrarenal defects, such as the VACTERL syndromes, which has the nonrandom pattern of vertebral abnormalities, anal atresia, cardiovascular defects, tracheoesophageal fistula, renal anomalies and limb defects. At least 3 of the defects need to be present for the present for the condition to be classified as VACTERL. The renal anomalies in VACTERL can vary in type and severity and may include hydronephrosis, ectopic kidneys, renal agenesis and dysplasia. There are some well-described single-gene defects that can include features of VACTERL. Holt-Oram and Smith-Lemli-Opitz syndromes are 2 such conditions, with patients with Holt-Oram syndrome having defects in limbs and heart due to mutation in transcription factor TBX5 and patients with Smith-Lemli-Opitz syndrome exhibiting facial anomalies, limb defects and developmental delay due to mutation in the DHCR7 gene. VACTERL is also sometimes associated with chromosomal anomaly trisomy 18.
Most cases of VACTERL syndrome, however, are not associated with other syndromes outside of the VACTERL spectrum. The etiology of most cases of VACTERL syndrome is not clear, but several gene have been identified to be involved with this phenotype, including mutations in mitochondial DNA, in the PTEN tumor-suppressor gene and in Fanconi anemia genes. This would to suggest that there are multiple potential genetic etiologies leading to a common constellation of defects and that VACTERL is not a single disease.
Wednesday, February 7, 2018
Ventilator part 3
PULMONARY EFFECTS
Barotrauma may result in pulmonary interstitial emphysema, pneumomediastinum, pneumoperitoneum, pneumothorax, and/or tension pneumothorax. High peak inflation pressures (>40cm water) are associated with an increased incidence of barotrauma. However, note that separating barotrauma from volutrauma is difficult, since increasing barometric pressure is usually accompanied by increasing alveolar volume.
Experimental models of high peak inflation pressures in animals with high extrathoracic pressures have not demonstrated direct alveolar damage from increased pressure without increased volume as well. Thus, the statement that high airway pressures result in alveolar overdistention (volutrauma) and accompanying increased microvascular permeability and parenchimal injury may be more accurate. Alveolar cellular dysfunction occurs with high airway pressures. The resultant surfactant depletion leads to atelectasis, which requires further increases in airway pressure to maintain lung volumes.
High-inspired concentration of oxygen result in free-radical formation and secondary cellular damage. The same high concsntratiom of oxygen can lead to alveolar nitogen washout and secondary absorption atelectasis.
It has been theorized that pulmonary biophysical and biomecahanical injury in the presence of bacterial lung infections contributes to bacterial translocation and bacteremia.
CARDIOVASCULAR EFFECTS
The heart, great vessels, and pulmonary vasculature lie within the chest cavity and are subject to the increased intrathoracic pressures associated with mechanical ventilation. The result is a decrease in cardiac output due to decreased venous return to the right heart (dominant), right ventricular dysfunction and altered left ventricular distensibility.
The decrease in cardiac output from reduction of right ventricular preload is more pronounced in the hypovolemic patient and in those with a low ejection fraction.
Exaggerated respiratory variation on the arterial pressure wave form is a clue that positive-pressure ventilation is significantly affecting venous return and cardiac output. In the absence of an arterial line, a good pulse oxymetri wave form can be equally instructive. A reduction in the variation after volume loading confirm this effect. These effects will most frequently be seen in patients with preload-dependent cardiac function (that is, operating on the right side of the Starling curve) and in hypovolemic patients or in those with otherwise compromised venous return.
Increased alveolar-capillary permeability secondary to pulmonary inflammatory changes may, alternatively, contribute to increased cardiac output.
RENAL, HEPATIC AND GASTROINTESTINAL EFFECTS
Positive-pressure ventilation is responsible for an overall decline in renal function with decreased urine volume and sodium excretion.
Hepatic function is adversely affected by decreased cardiac output, increased hepatic vascular resistance and elevated bile duct pressure.
The gastric mucosa does not have autoregulatory capability. Thus, mucosal ischaemia and secondary bleeding may result from decreased cardiac output and increased gastric venous pressure.
Tuesday, February 6, 2018
Ventilator part 2
METHODS OF VENTILATORY SUPPORT
- Continous mandatory ventilation
- Assist-control ventilation
- Intermittent mandatory ventilation
- Synchronous intermittent mandatory ventilation
The initial choice of ventilation mode is institution and practitioner dependent. Assist-contrl ventilation, as in continuous mandatory ventilation, is a full support mode in that the ventilator performs most, if not all, of the work of breathing. These modes are beneficial for patients who require a high minute ventilation. Full support reduces oxygen consumption and carbon dioxide production of the respiratory muscles. A potential drawback of assist-control ventilation in the patient with obstructive airway disease is worsening of air trapping and breath stacking.
When full respiratory support is necessary for the paralyzed patient following neuromuscular blockade, no difference exists in minute ventilation or airway pressures with any of the above modes of ventilation.
Ventilator part 1
Intubation, with subsequent mechanical ventilation, is a common life-saving intervention in emergency departement. Many different strategies of positive-pressure ventilation are available. These are based on various permutations of triggered volume-cycled and pressure-cycled ventilations and are delivered at a range of rates, volume and pressures. Poor ventilatory management can inflict serious pulmonary and extrapulmonary damage that may not be immediately apparent,
Because many of the effects of ventilator-induced lung injury are delayed and not seen while patients are in the emergency fepartement, much of our understanding of the adverse consequences of volume trauma, air-trapping, baro trauma and oxygen toxicity has come from the critical care literature.
While the fundamental principles underlying mechanical ventilatory support have changed little over the decades, much progress has been made in our understanding of the secondary pathophysiologic changes associated with positive-pressure ventilation.
Ventilatory strategies have been devised for different disease processes to protect pulmonary parenchyma while maintaining adequate gas exchange, and they may be responsible for the increased rates of survival for pathologies such as acute respiratory distress syndrome.
MODES OF MECHANICAL VENTILATION
- Volume-cycled mode
Because the volume-cycled mode ensures a constant minute ventilation despite potentially abnormal lung compliance, it is a common choice as an initial ventilatory mode in the emergency departement. A major disadventage is that high airway pressures may be generated, potentially resulting in barotrauma. Close monitoring and use of pressure limits are helpful in avoiding this problem. Note that ventilators set to volume-cycled mode function well as monitors of patients pulmonary compliance, which will be decreased in physiological states such as worsening acute respiratory distress syndrome, pneumothorax, right mainstem intubation, chest wall rigidity, increased intra-abdominal pressure and psychomotor agitation. These pathophysiological states increase peak pressure and should be considered whenever pressure alarms are sounded.
- Pressure-cycled mode
A theorical adventage of pressured-cycled modes is a decelerating inspiratory flow pattern, in which inspiratory flow tapers off as the lung inflates. This usually results in a more homogeneous gas distribution throughout the lungs. However, no definite evidence exists that this results in a reduction of the rate of ventilator-induced lung injury or overall mortality. Nevertheless, pressured-cycled ventilation has achieved considerable popularity in the intensive care setting for management of patients with acute respiratory distress syndomes, whose lungs are most likely to be characterized by a broad range of alveolar dysfuction and are also most vulnerable to the effects of barotrauma and volutrauma.
A major disadvantage is that dynamic changes in pulmonary mechanics may result in varying tidal volumes. This necessitates close monitoring of minute ventilation and limits the usefulness of this mode in many emergency departement patients. However, newer ventilators can provide volume-assured pressure-cycled ventilation, which incresae peak pressures as needed to deliver a preset minimum tidal volume.
Monday, February 5, 2018
Congenital Lung Abnormalities
CONGENITAL PULMONARY AIRWAY MALFORMATION
Congenital pulmonary airway malformation are a heterogenous group of cystic and non cystic lung lesion that largely result from early airway maldevelopment. Congenital pulmonary airway malformation may communicate with the proximal airways. although this communicationis abnormal. Most of them derive their blood supply from the pulmonary artery and drain via pulmonary veins, with the exception of hybrid lesions, which can have a systemic blood supply.
A fast growing congenital pulmonary airway malformation may cause mediastinal shift and subsequent development of polyhydramnions and hydros. It well established that indicators of a poor prognosis include large lesions, bilateral lung involvement and hydrops.
If not recognized antenatally, congenital pulmonary airway malformation are usually discovered between the neonatal period and 2 years of age, manifestating as respiratory difficulty or infection. Symptomatic infants who are diagnosed postnatally are treated with surgical resection, which generally consists of lobectomy or segmental resection.
CONGENITAL LOBAR OVERINFLATION
Congenital lobar overinflation also referred to as congenital lobar emphysema, is characterized by progressiive lobar overexpansion, usually with compression of the remaining (ipsilateral) lung. The underlying cause can be secondary to an intrinsic cartilaginous abnormality with resultant weak or absent bronchial cartilage, extrinsic compression of an airway (eg, by a large pulmonary artery or a bronchogenic cyst). In either case, the collapsed airway can act as one-way valve, resulting in air trapping. Although the alveoli expand, the alveolar walls remain intact, therefore, the term emohysema is technically inaccurate.
Although most patients with congenital lobar overinflation present in the neonatal period, typically with respiratory distress, congenital lobar overinflation can be detected in utero by ultrasonography and magnetic resonance imaging.
BRONCHIAL ATRESIA
Bronchial atresia is a rare anomally resulting from focal obliterasion of a segmental, subsegmental or lobar bronchus. The bronchi distal to the stenosis are dilated and filled with mucus, with mild hyperinflation of the adjacent lung due to collateral air drift. In bronchial atresia, the airway is occluded rather than narrowed, consequently, there is no ball-valve effect as seen in congenital lobar overinflation. Hence, the lobe or segment does not become nearly as hyperinflated as with congenital lobar overinflation. This may be the reason why congenital lobar overinflation manifest in the neonatal periods, whereas bronchial atresia is usually found incidently in adults.
BRONCHOGENIC CYSTS
Bronchogenic cysts are part of the spectrum of foregut duplication cysts and are developmental lesion resulting from abnormal ventral budding of the tracheobronchial tree, probably occuring between the 26th and 40th days of fetal life. They are mostly situated in the mediastinum, typically near the carina. Less commonly, they may occur within the lung parenchyma, pleura or diaphragm
Most bronchogenic cysts are found incidentally. In infants, symptoms are generally caused by compression of the trachea or bronchii and esophagus, leading to wheezing, stridor, dyspnea and dysphagia. Intraparenchymal cysts may manifest with recurrent infection. Symptomatic cysts are generally resected surgycally.
Pulmonary underdevelopment
Pulmonary underdevelopment has been classified into three categories:
- Pulmonary agenesis
- Pulmonary aplasia
- Pulmonary hypoplasia
It has been hypothesized that abnormal blood flow in the dorsal aortic arch during the 4th week of gestation (embryonic phase) causes pulmonary agenesis. Unilateral pulmonary agenesis is difficult to diagnose with prenatal ultrasonography;, however, it can be suspected on the basis of mediastinal shift. More than 50% of affected fetuses have other abnormalities involving the cardiovascular (patent ductus arteriosus, patent foramen ovale), gastrointestinal (tracheoesophageal fistula, imperforate anus), genitourinary, or skeletal (limb anomalies, vertebral segmentation anomalies) system. Imaging findings in pulmonary aplasia and agenesis are similar, except for the presence of short blind ending bronchus in aplasia. Postnatal radiography demonstrates diffuse opacification of the involved hemythorax with ipsilateral mediastinal shift and computed tomography helps confirm the absence of the lung parenchyma, bronchus and pulmonary artery on the involved side.
Pulmonary hypoplasia can be primary or secondary. Primary pulmonary hypoplasia, in wich a cause cannot be elucidated , is much less common than secondary hypoplasia. The majority of cases of pulmonary hypoplasia are secondary to a process limiting the thoracic space for lung development, which can be either intrathoracis or extrathoracic. The most common intrathoracic cause is congenital diaphragmatic hernia, which is left sided in 75-90% of cases, right sided in 10%, and bilateral in 5%. Left-sided congenital diaphragmatic hernia is relatively easier to detect due to the presence of an identifiable fluid-filled stomach in the thorax. In right-sided congenital diaphragmatic hernia, the liver herniates into the chest, which may be difficult to detect due to the solid echotexture of the liver. The herniated liver can be confused with a mass originating in the lung. Color Doppler imaging maybe helpful in identifying the portal and hepatic veins. Magnetic resonance imaging provides greater soft tissue contrast, which is useful in assessing the size of the hernia and the location of other abdominal viscera. Other intrathoracic causes of the pulmonary hypoplasia include congenital pulmonary airway malformations, broncho-pulmonary sequestrations, a cardiac or mediastinal mass, lymphatic malformations, and agenesis of the diaphragm.
The most common extrathoracic cause of pulmonary hypoplasia is severe oligohydramnions, occuring secondary to either fetal genitourinary anomalies such as renal agenesis, cystic renal dysplasia and urinary tract obstruction or prolonged rupture of membranes. A hypoplastic thorax, occurs in skeletal dysplasias, such as thanatophoric dysplasia or Jeune syndrome, in wich a small and rigid thoracic cage causes pulmonary hypoplasia. Antenatally, thoracic circumference measurements are obtained in an axial plane at the level of the four-chamber view of the heart.
Normal anatomy of the fetal thorax
At ultrasonography, the fetal lungs normally appear homogenous and are slightly more echogenic than the liver. The echogenicity of the lung increases as gestation advances. The presence of cysts or focal increased echogenicity of the lung parenchyma indicates a mass. On the four-chamber view, the heart occupies 25-30% of the thoracic volume and is positioned in the left anterior quadrant, just to the left of midline. The axis of the heart is determined relative to the interventricular septum, which makes an angle of 450 with the midline. Cardiomediastinal shift may often be the first clue to the presence of unilateral chest mass or diaphragmatic hernia. Hence, the four chamber view of the heart is an important landmark in the fetal chest and should be visualized in all fetuses during 2nd and 3rd trimesters as part of routine obstetric imaging. Fetal lung volumes can be measured with three and four dimensional ultrasonography and should be calculated in fetuses with lung abnormalities for the estimation of residual lung volume.
At magnetic resonance imaging, the trachea, bronchi and lung demonstrate high T2 signal intensity relative to the chest wall muscles, since they contain a significance amount of fluid. As the lungs mature, there is increasing production of alveolar fluid, thereby increasing production of alveolar fluid, thereby increasing the signal intensity of the lungs relative to the liver. Normal lung volumes can be calculated with magnetic resonance imaging as well and have been shown to increase with gestational age.
Congenital Lung Abnormality
Congenital lung abnormalities are being detected more frequently at routine high-resolution prenatal ultrasonography. The most commonly encountered anomalies include lung agenesis-hypoplasia compllex (pulmonary underdevelopment), congenital pulmonary airway malformation, congenital lobar overinflation, bronchial atresia, bronchogenic cyst, congenital high airway obstruction syndrome, scimitar syndrome and bronchopulmonary sequestration. Recognizing the antenatal and postnatal imaging features of these abnormalities is necessary for optimal prenatal counceling and appropriate perinatal and postnatal management.
With advances in both fetal ultrasonography and magneting resonance imaging, abnormalities of the thorax are increasingly being recognized antenatally, allowing providers to anticipate managementy issues at the time of delivery or later in neonatal life, and help parents comprehend the prognosis. Recognizing the imaging features of a variety of intra thoracic processes in the fetus is necessary for appropriate guidance of the clinicians caring for the mother.
Congenital thoracic anomalies range from abnormal lung with normal vasculature (e.g, congenital lobar over inflation) to abnormal vasculature with normal lung (e.g, hypogenetic lung syndrome, bronchopulmonary sequestration). The vascular abnormality (e.g, absent pulmonary artery associated with pulmonary agenesis) may be the underlying cause of the malformation).
The most commonly encountered anomalies can be classified into three broad categories: bronchopulmonary (lung bud) anomalies, vascular anomalies and combine lung and vascular anomalies. Bronchopulmonary anomalies include lung agenesis-hypoplasia complex (pulmonary underdevelopment), congenital pulmonary airway malformations, congenital lobar overinflation, bronchial atresia and bronchogenic cysts. Vascular anomalies include absence of the main pulmonary artery, anomalous origin of the left pulmonary artery or pulmonary sling, anomalous pulmonary venous drainage and pulmonary arteriovenous malformations. Combined lung and and vascular anomalies include scimitar syndrome and bronchopulmonary sequestration. Vascular abnormalities may accompany bronchopulmonary abnormalities in some cases: for example, pulmonary vascular abnormalities with pulmonary hypoplasia or agenesis, or a systemic arterial supply to a small cysts congenital pulmonary airway malformations.
Understanding the pathogenesis of broncho-pulmonary malformations is difficult and confusing. according to one long-held theory, many of these lesions are due to defective foregut budding and differentiation. Another theory of causation is that these lesions are related to airway obstruction with secondary pulmonary dysplastic
Mechanism of respiration
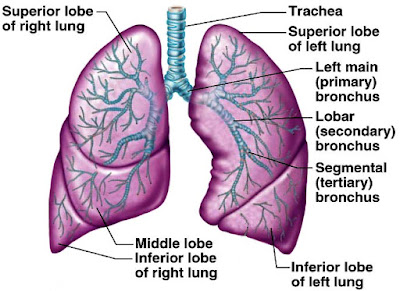
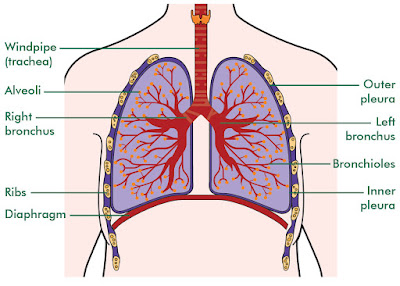
Air containing oxygen enters the body through the nose and mouth. From there it passes through the pharix or throat on its way to the trachea (windpipe). The trachea divides into two main airways called bronchi upon reaching the lungs; one bronchus serves the right lung and the other the left. The bronchi subdivide several times into smaller bronchi, which then divide into smaller and smaller branches called bronchioles. These bronchi and bronchioles are called the bronchial tree because the subdividing that occurs is similar to the branching of an inverted tree. After a total of about 23 division, the bronchioles end at alveolar ducts. At the end of each alveolar duct, are clusters of alveoli (air sacs). The oxygen transported through the respiratory system is finally transferred to the bloodstream at the alveoli,
The trachea, main bronchii, and approximately the first dozen divisions of smaller bronchi have either rings or patches of cartilage in their walls that keeps them from collapsing or blocking the flow of air. The remaining bronchioles and the alveoli do not have cartilage and are very elastic. This allows them to respond to pressure changes as the lungs expand and contract.
Blood vessels from the pulmonary arterial system accompany the bronchi and bronchioles. The blood vessels also branch into smaller and smaller unit ending with capillaries, which are in direct contact with each alveolus. Gas exchange occurs through this alveolar-capillary membrane as oxygen moves into and carbon dioxide moves out of the bloodstream. Although the 300 million alveoli found in the lungs are microscopic, they have a total surface area equivalent to the size of a tennis court.
Diffusing capacity measures the ease with which gas exchange takes place between the alveoli and capillaries. Certain lung diseases affecting the alveoli and capillary walls can interfere with diffusion and reduce the amount of oxygen reaching the bloodstream.
The movement of air into and out of the lungs is called ventilation. The contraction of the inspiratory muscles (principal inspiratory muscle is the diaphragm) causes the chest cavity to expand, creating a negative pressure. The resulting flow of air into the lungs is called inspiration. During the maximal inspiration, the diaphragn contracts forcing the abdominal contents downwards and outwards. The external intercoastal muscles, found between the ribs, are also involved. The muscles contract and raise the ribs during inspiration, thus increasing the diameter of the chest cavity. In addition to these muscles, the scalene muscle and the sternomastoid muscle in the neck may be employed during extreme ventilation or in condition of respiratory distress.
Normal expiration is passive process resulting from the natural recoil or elasticity of the expanded lung and chest wall (however, when breathing is rapid, the internal intercoastal muscles and abdominal muscles contract to help force air out of the lungs more fully and quickly). A lung can be viewed as the opposite of a sponge. When a sponge squeezed and released, its elasticity causes it to rebound to its larger initial size. At the end of an inspiration, the elasticity of the lung causes it to return to its smaller inter-breath size. The ability of the lung to do this called elastic recoil.
The degree of stiffness or compliance of the lung tissue affects the amount of pressure needed to increase or decrease the volume of the lung. Lung compliance can affect elastic recoil. With increasing stiffness, the lung becomes less able to return to its normal size during expiration.
The amount of air flow resistance can also affect lung volumes. Resistance is the degree of ease in which air can pass through the airways. It is determined by the number, length and diameter of the airways. An individual with a high degree of resistance may not be able to exhale fully, thus some air becomes trapped in the lungs.
The Respiratory System
A person can live for weeks without food and a few days without water but only few minutes without oxygen. Every cell in the body needs a constant supply of oxygen to produce energy to grow, repair or replace itself, and maintain vital functions. The oxygen must be provided to the cells in a way that they can use. It must be brought into the body as air that is cleaned, cooled or heated, humidified and delivered at the right amount.
The respiratory system is the body's link to this supply of life-giving oxygen. It includes the diaphragm and chest muscle, the nose and mouth, the pharynx and trachea, the bronchial tree, and the lungs. The bloodstream, the heart and the brain are also involved. The bloodstream takes oxygen from the lungs to the rest of the body and returns carbon dioxide to them to removed. The heart creates the force to move the blood at the right speed and pressure throughout the body. The smooth functioning of the entire system is directed by the brain and the autonomic nervous syatem.
A person at rest breathes about 6 liter of air a minute. Heavy exercise can increase the amount to over 75 liters per minute. During 8-hour work day of moderate activity, the amount of air breathed may be as much as 8.5 m3 .The skin, with its surface area of approximately 1,9 m2 is commonly thought to have the greatest exposure to air of any body part. However, in reality the lungs have the greatest exposure, with a surface area exposed to air of 28 m2 at rest and up to 93 m2 during a deep breath.
The respiratory system is susceptible to damage caused by inhaled toxic materials and irritants because the surface area of the lungs exposed to the air is so large and the body's need for oxygen so great. The ability of the respiratory system to function properly has a great impact on the body. Disease in any one of its parts can lead to disease or damage to other vital organs.
Lung Development part 4
There are a number of physical influences on lung growth. Proper development of the lung is dependent of the presence of both lung liquid and amniotic fluid. The lung liquid is secreted by pulmonary epithelium. The volume of lung fluid is maintained by the activity of the upper airway which acts as a gatekeeper by controlling the resistance to efflux of fluid out of the lung and trachea during non-breathing periods, and by diaphragmatic movement associated with fetal breathing movements. The larinx is the major site of regulation of efflux and therefore of lung liquid volume. During fetal breathing movements, when the upper airway resistance is decreased, diaphargmatic movements help to maintain lung liquid volume. The experimental drainage of lung liquid leads pulmonary hypoplasia.
Amniotic fluid is also required for normal lung development. Amniotic fluid originates in the lung and fetal kidney. Oligohydramnions is associated with lung hypoplasia (Potter syndrome-renal agenesis, lack of fetal urine). The mechanism of lung hypoplasia in this syndrome is uncertain but perhaps it is due to increased efflux of lung liquid into the amniotic space.
Lung hypoplasia may caused by a number of other factors that restrict or better, compress the fetal lung. Condition or lesions known to cause lung hypoplasia are:
- Congenital diaphragmatic hernia, in which usually the left hemithorax is occupied by intestinal contents
- Musculosceletal abnormalities of the thorax which do not allow full expansion of the thoracic cage
- Space occupying lesions of thorax, such as fetal pleural effussions
- Changes in geometry due to oligohydramnions associated with renal or urinary tract abnormalities
Surfactant decreases surface tension within alveoli and prevents collapse of alveoli during exhalation. In the absence of surfactant, the alveolus would be unstable and collapse at the end of each breath. Tremendous work would be required to open up the alveolus with each breath. In fact, a mutation in the gene coding for surfaactant protein B leading to an absence of this crucial protein is an extremely rare cause of respiratory failure in terms newborns.
Type II cells and their associated surfactant can develop in the presence of pulmonary hypoplasia. Gas exchange is possibly by 26-27 weeks, though not necessarily sustainable. Surfactant production gradually increases with advancing gestational age. The surfactant system matures by 36 weeks in most fetuses.. Production of surfactant by type II cells is hormonally influenced. Corticotrophin stimulates lung maturation via cortisol. Cortisol induces fetal lung fibroblasts to produce fibroblast pneumocyte factor which stimulates surfactant production in type II cells. Thyroid hormones are also required for development of the surfactant system. At the time of birth, epinephrine and arginin vasopressin suppress fetal lung liquid formation, and play a role in its rearbsobtion. These two hormones are in turn dependent on increasing concentrations of glucocorticoids that occur at term.
The development of the surfactant system, to the point that spontaneous respiratory function can occur, usually trakes place by 36 weeks of gestation. Birth before 36 weeks may be associated with respiratory compromise and failure. The incidence and severity of the lung disease is greater to the degree of prematurity. A number of strategies have been developed to try to deal with this problem, including the use of antenatal steroids to promote lung maturity, the use of various forms of positive pressure to maintain the lungs in the open state, and the administration of exogenous surfactant.
Lung Development part 3
The development of the pulmonary arterial system follows a similar progression to that of the developing airways. Development of, and branching of the pulmonary artery mirrors bronchial branching, and later mirrors alveolar development. By the beginning of the cannalicular stage arteries in the pre acinar region have formed. The development of muscle within the wall of the pulmonary blood vessels lags behind structural development. Muscularization of intra-acinar arteries does not keep pace with the appearance of new arteries and is not complete until childhood. What is the significance of this? Although the control of the pulmonary blood flow in terms of distribution within the lung is controlled by a number of factors (physical location of lung units, gravity, oxygen, nitric oxide), alveolar oxygen tension is probably the most important determinant of pulmonary blood flow. The ultimate effector governing the distribution of pulmonary blood flow is pulmonary vascular muscle. The process of hypoxic pulmonary vasoconstriction governs this distribution of blood flow. Decreased alveolar oxygen tension will caused vasoconstriction in the area of the alveolus with a decreased oxygen tension, thus diverting blood to better oxygenated areas of the lung. During a generalized decrease in oxygen tension, or hypoxia, there is vasoconstriction of pulmonary blood vessels throughout the lung which causes a rise in pulmonary artery pressure.
Why is there is so little blood flow in pulmonary artery in the fetus, and why does this suddenly change with the first breath after birth? The fetal alveolus is filled with liquid not exposed to the atmosphere, therefore oxygen tension in the alveolus is very low. As a consequence of this low oxygen tension in the alveolus, there is generalized pulmonary vasoconstriction, arise in pulmonary artery pressure, and diversion of blood flow from the pulmonary bed, across the ductus arteriosus to the systemic circulation. At birth, as the alveoli become gas filled, and oxygen tension in the alveolus rises, vasoconstriction decreases, and blood flow now increases to the lung. The mediator of the vasodilatation in the pulmonary bed is nitric oxide. Pulmonary artery pressure is decreases after birth. In a small number of neonatus the nitric oxide system in the pulmonary vascular bed is dysfunctional and pulmonary arterial vasodilatation does not occur after birth. When this is occurs pulmonary artery pressure remain elevated, the ductus arteriosus remain open, blood with low oxygen tension is shunted into the systemic circulation (a situation analogous to that found in the fetus) and systemic oxygen delivery is compromised. This syndrome, originally called persistent fetal circulation, is now called persistent pulmonary hypertension of the newborn.
Thursday, February 1, 2018
Lung Development part 2
MATURATION OF THE LUNG :
- Psedoglandular stage
During this stage, the first differentiation of lung epithelium occurs. By 13 weeks cilia appear in the proximal airways. Mesenchyme is necessary for this epithelium differentiation to occur and there is a transition from formation of bronchial epithelial cells (ciliated columnar and goblet cells) to alveolar type II cells. Conversely, the differentiation of lung mesenchyme requires the presence of lung epithelium.
- The canalicular stage
- The terminal or saccular stage
At birth, the air-containing space, later to become the alveolus, has called a primitive saccule. There are approximately 20x106 saccules at birth.
- Post natal or alveolar stage
Subscribe to:
Posts (Atom)