Wednesday, September 4, 2019
STEM CELL part 27
There is obviously a moral obligation to provide new and better treatments for patients. But there are also obstacles on the road, regulatory as well as technical. If the problem of standardization, in particular of induced pluripotent stem cell lines, is not addressed, this will create regulatory hurdles as long as the FDA regards every cell line as a new treatment. Moreover, if cell therapies are to be commercially successful and affordable, solutions to the problem of scaling up have to be found.
(Goran Hermeren, Prof. em. Medical ethics, Lund University)
STEM CELL part 26
Changes in the scientific landscape may bring about changes in the ethical landscape. Neither science nor values are static. Ongoing dialogues between different stakeholder groups on how to address the ethical issues raised by different therapies is a long term investment which will pay off, particularly if the decisions taken are regarded as preliminary and updated if and when there is new evidence or changes in the landscape of values.
(Goran Hermeren, Prof. em. Medical ethics, Lund University)
STEM CELL part 25
Finally, it is clear that all types of stem cell research, including embryonic stem cell and induced pluripotent stem cell research, must take place within a carefully considered ethical and regulatory framework, and that therapies based on living cells pose new challenges for regulators.
STEM CELL part 24
Broader ethical questions include tissue ownership, informed consent when donating cells for stem cell banking, patient safety and data protection, and access to treatments (Hug and Hermeren, 2011, Hermeren, 2012)
STEM CELL part 23
The discovery of induced pluripoten tstem cells raised the possibility that embryonic stem cells research would no longer be necessary, there be circumventing the ethical issues present in embryonic research. To date, this has not been the case: the stem cell field continues to rely both embryonic stem cells and induced pluripotent stem cell research to progress the understanding of plurypotency and its potential applications (Smith and Blackburn, 2012). Further, it has become clear that induced pluripotent cell research is not free of ethical considerations (Hug and Hermeren, 2011). For instance, the potential of these cells to generate sperm and egg cells, or even a whole new individual, raises new ethical questions about the status of the cells themselves and how they may be used.
STEM CELL part 22
In Germany, no new human embryonic stem cell lines can be generated, but research using imported lines generated prior to May 1, 2007 is permitted, while in the USA, a series of restrictions implemented between 1995 and 2009 limited federal funding for human embryonic stem cell research. The patentability of human embryonic stem cells and lines is similarly complex (The Hinxton Group, 2013). Some countries, including the US, place liitle or no restriction on this practice, while in 2011 the European Court of Justice ruled that patents cannot be granted in Europe for any technologies based on research using human embryonic stem cells.
STEM CELL part 21
Ethics, policy and regulation
Like many areas of biomedical science, stem cell research has provoked debate regarding the ethics and regulation of the research and resulting therapies. Initiallly these discussions focused largely on the moral status of the embryo (EuroStemCell, 2011). Goverments responded with different regulations and legislations, leading to international complexities (Kawakami et al., 2010, Nakatsuji et al., 2007, STEMGEN, 2013). Countries including Australia, Singapore, Spain, South Korea, Belgium, the UK, and Sweden take a tightly regulated but permissive approach to research involving the use of human embryos to generate embryonic stem cell lines. Other have placed some restrictions on research in this area, either through direct legislation or by limiting the uses of research funding.
STEM CELL part 20
Finally, where use of a patient's own cells is not possible, either because a sufficient number of cells cannot be obtained or because protocols for generating the required cell type in the lab (e.g., from induced pluripotent stem cells) have not yet been developed, the consequences of immunosupprtession must also be considered. Banking human embryonic stem cell and induced pluripotent stem cell lines is one way to ensure that patients can receive cells with a good immunological match, thus minimizing any required immunosuppression (Turner et al., 2013)
STEM CELL part 19
The ability to functionally integrate transplanted cells into damaged organs is also a major challenge. For some cell types, such as pancreatic beta cells and retinal pigment epithelial cells, it appears that transplantation of the cells alone will be sufficient to ameliorate symptoms or cure the disease (in these examples, diabetes and macular degeneration, respectively). For others, it is likely that complex organ structure creation through in vitro tissue engineering will be required. Here, the challenge is not only to derive the correct organ structure at scale, but also to maintain long-term function following transplantation. To achieve this will require new collaboration between tissue engineers and stem cell and vascular biologist, as well as improved understanding of how stem cells are controlled by their specific environment (niche) within the body.
STEM CELL part 18
Biological challenges
While cell replacement offers hope for the treatment of many diseases in the long term, it may still be some time before large-scele clinical use is available for most applications. Understanding how to produce many of the speialized cel types in vitro remains a major hurdle. Furthermore, the field faces challenges around quality control. It is essential that only defined cell populations are introduced into patients; this requires careful characterization of the cell populations intended for transplantation, in terms of gene expression and epigenetic profiles and functional attributes, and also to ensure that the populations do not contain other potentially harmful cell types. For cells generated from human pluripotent cells, for example, contamination of the transplant population with even a small number of residual embryonic stem cell or induced pluripotent stem cells could promote tumor formation. Additionally, as cells can acquire mutations during the culture process, stringent quality control is essential to ensure that cultured cells intended for transplantation have not acquired undesirable properties.
STEM CELL part 17
Regenerative therapies
In addition to cell replacement strategies, increased understanding of the intrinsic regenerative potential of individual organs, coupled with knowledge of how to control the scarring response in damaged tissues, may allow the development of drugs aimed at stimulating the body's own (endogenous) stem cells to initiate or enhance repair. This approach is expected to prove more suitable than cell replacement for some diseases.
STEM CELL part 16
Cell replacement therapies
Stem cell research is also anticipated to contribute to new cell-based therapies through the use of cells generated from embryonic stem cells and/or induced pluripotent stem cells, or of ex vivo tissue stem cells, to replace missing or damaged cells, and (in the future) to generate artificial organs for transplantation. Although is not yet possible to generate many cell types in the lab, or to expand many tissue stem cell types ex vivo, clinical trials using human fetal and adult cells, as well as human embryonic stem cells and induced pluripotent stem cell-derived cells, are already in progress or on the horizon. For example, retinal pigmen epithelial cells have been produced from both human embryonic stem cells and human induced pluripotent stem cells (Carr et al., 2013, Jin et al., 2009), and both cases are conducting clinical trials to test the capacity of these pluripotent cell-derived cells to treat macular degeneration.
STEM CELL part 15
Some progress has been made towards these goals. For instance, in recent tests human embryonic stem cell-derived hepatocytes performed as well as the current FDA gold standard primary adult cells at predicting human hepatotoxicity (Szkolnicka D et al., 2014). However, the ability to control the differentiation of both tissue and pluripotent stem cells remains a challenge for the field.
STEM CELL part 14
Induced pluripotent stem cell technology has also made it possible to conduct parallel high-throughput compound screens on defined cell types derived from a large number of different individuals. This technology will allow the screening process to account for genetic differences in the response to potential new drugs. As induced pluripotent stem cells can now be easily generated from patients, including those with inherited diseases and their unaffected relatives, they also provide a new way to investigate the molecular basis of disease-prone cells side-by-side in the lab, enabling the development of improved pharmaceutical interventions.
STEM CELL part 13
Stem cells in drug discovery, toxicity testing, and disease modelling
Stem cell research has the potential to improve and accelerate drug screening, drug discovery, and pre-clinical toxicological assessment of new drugs. Controlled differentiation of human pluripotent cells and/or ex vivo expansion of human tissue stem cells could produce unlimited supplies of defined human cell types. Once developed, this technology should permit screening of more compounds in shorter time and at less expense than is currently possible. Additionally, as it will allow primary screens to be conducted on human cells, it may reduce the number of promising drugs that fail in late phase II/III clinical trials because of unexpected differences between animals and humans, as well as the number of animal tests needed.
STEM CELL part 12
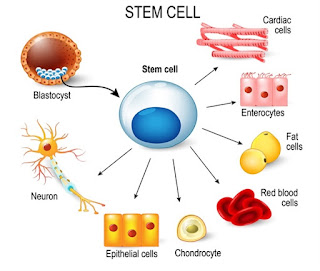
Three key facts about stem cells:
- The defining characteristic of a stem cell is that it can self-renew or differentiate
- Stem cells enable the body to grow, repair and renew
- There are three types of stem cells (tissue stem cells, embryonic stem cells, induced pluripotent stem cells)
STEM CELL part 11
Subsequent advances, including derivation of human stem cell lines and the advent of human induced pluripotent stem cell technology, as well as progress in making specific specialized cells from stem cells in the laboratory, have suggested that stem cell therapies may be more broadly applied to aid a wide range of disorders.
STEM CELL part 10
In hematopoietic stem cell transplantation, stem cells are harvested from the patient or donor and, following leukemia treatment, are transplanted back into the patient to restore their blood and immune systems. In the case of stem cell-based skin or corneal grafting, skin or limbal stem cells are obtained from the patient, and then grown in the lab to produce sheets of cells sufficient to cover the burn or wound area. These applications exemplify two different approaches to transplanting tissue stem cells: one requires expanding cell numbers through lab culture, while the other does not.
STEM CELL part 9
Tissue stem cells have been used therapeutically for many years in the contexts of hematopoietic stem cell transplantation, a vital component in the sucessful therapy of many types of blood cancer; stem cell-based skin grafting (Green et al., 1979, Green, 1989), which can save the lives of patients with extensive third-degree burns; and limbal stem cell grafting, which can restore sight to patients with impaired vision caused by corneal damage (Rama et al., 2010).
STEM CELL part 8
Induced pluripotent stem cells
Induced pluripotent stem cells were discovered in 2006 using mouse cells (Takahashi and Yamanaka, 2006); just a year later, this finding was replicated in human cells (Takahashi et al., 2007, Yu et al., 2007). Induced pluripotent stem cells are generated from specialized cells by using a technique called "reprogramming". This groundbreaking work was awarded the Nobel Prize in Physiology or Medicine in 2012. Researchers have rapidly adopted induced pluripotent stem cells for study, although there is on going discussion in the field about whether they are completely interchangeable with embryonic stem cells (Yamanaka, 2012)
STEM CELL part 7
Epiblast stem cells
Epiblast stem cells are a type of pluripotent mouse stem cells derived from a slightly later stage of embryonic development than mouse embryonic stem cells; they are more closely resemble the human embryonic stem cells (Tesar et al., 2007, Brons et al., 2007)
STEM CELL part 6
Embryonic stem cells
Embryonic stem cells are derived from early-stage, pre-implantation embryos, and were the first type of pluripotent stem cells to be discovered: first in mice (Evans and Kaufman, 1981, Martin, 1981) and then in humans (Thomson et al., 1998) and several additional species.
STEM CELL part 5
There are currently three types of pluripotent stem cells, each generated by a different route:
- Embryonic stem cells
- Epiblast stem cells
- Induced pluripotent cells
STEM CELL part 4
Pluripotent stem cells, in contrast, have the potential to generate any type of cell found in the body. Pluripotent stem cells are generated in the laboratory by capturing or recreating cell types that exist only transiently during embryonic development, and have not been identified in the adult body.
STEM CELL part 3
Tissue (or adult) stem cells are found throughout the body, where they function to maintain the organ or tissue in which they reside, throughout the lifespan. Most rapidly renewing tissues are maintained by stem cells, with the notable exception of the liver, which is maintained by specialized liver cells called hepatocytes. Under normal physiological conditions, each type of tissue stem cell only generates cells of the organ or tissue system to which it belongs: the blood (hematopoietic) stem cell generates blood, the skin stem cell generates skin, and so on. An exception is the mesenchymal stem cell, which can generate bone, cartilage, and muscle; however, while the mesenchymal stem cell field has generated much valuable research, it has also attracted controversy.
STEM CELL part 2
Beyond this primary definition, stem cells are classified into two major sub-types, based on the range of specialized cells they can generate :
- Tissue (or adult) stem cells
- Pluripotent stem cells
STEM CELL part 1
Stem cells, whether they occur in the body or in the lab, are defined by two cardinal properties: they can self-renew (generate perfect copies of themselves upon division) and differentiate (produce specialized cell types that perform specific functions in the body). The promise of stem cells as new tools for benefiting human health resides in these twin properties that, in principle, allow production of unlimited quantities of defined cell types (e.g., for use in drug screening or transplantation)
Subscribe to:
Posts (Atom)