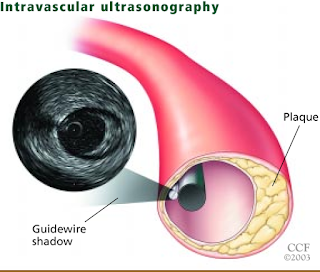
What will I experience during and after the procedure?
You will feel a slight pin prick when the needle is inserted into your vein for the intravenous line (IV) and when the local anesthetic is injected. Most of the sensation is at the skin incision site, which is numbed using local anesthetic. You may feel pressure when the catheter is inserted into the vein or artery.
If the procedure is done with sedation, the intravenous (IV) sedative will make you feel relaxed, sleepy and comfortable for the procedure. You may or may not remain awake, depending on how deeply you are sedated.
You may feel slight pressure when the catheter is inserted, but no serious discomfort.
You will not feel the catheter in your artery and you will not feel any pain during acquisition of the ultrasound images.
You may need to lie flat on your back with pressure applied to the catheter insertion site for a few hours after the test to or prevent bleeding. In some cases, your physician may use a device that seals the small hole in the artery, called a "closure device," which will allow you to move around sooner.
For several hours, your catheter site will be checked for bleeding or swelling and your blood pressure and heart rate will be monitored. Your physician may prescribe medication to relax your arteries to protect against spasm of the arteries or to prevent blood clots.
You may feel a little sleepy until the sedative has worn off
Your time in the hospital will vary depending on whether intravascular ultrasound was done in conjunction with another procedure such as catheter angiography or angioplasty. While intravascular ultrasound itself does not add to your recovery time, catheter angiography recovery will require you to stay in the hospital for observation for up to six hours. Angioplasty and vascular stenting recovery may require 12 to 24 hours.
After you return home, you should rest and drink plenty of fluids. Avoid lifting heavy objects and strenuous exercise at least 24 hours. It is strongly recomended that you quit smoking as this is a major cause of atherosklerosis.
The catheter insertion site may be bruised and sore. If bleeding begin where the catheter was inserted, you should lie down, apply pressure to the site and call your physician.
Call your physician immediately if you notice any change in the color of your leg, pain, swelling or warm feeling in the area where the catheter was inserted.