Thursday, July 12, 2018
DIURETICS
Concentrated urine
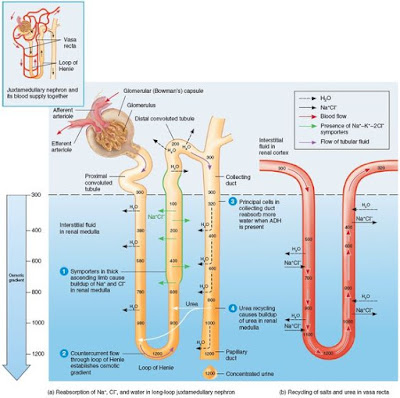
The formation of concentrated urine depends on the medullary osmotic gradient and the presence of antidiuretic hormone. In the distal tubules, the filtrate osmolality is approximately 100 mOsm, but as the filtrate flows through the collecting ducts and is subjected to the hyperosmolar conditions in the medulla, water rapidly leaves the filtrate, followed by urea. Depending on the amount of antidiuretic hormone released (which is keyed to the level of body hydration), urine concentration may rise as high as 1200 mOsm, the concentration of the interstitial fluid in the deepest part of the medulla. With maximal antidiuretic hormone secretion, up to 99% of the water in the filtrate is reabsorbed and returned to the blood, and a half liter per day of highly concentrated urine is excreted. The ability of kidneys to produce such concentrated urine is critically tied to ability to survive without water. Water reabsorption that depends on the presence of antidiuretic hormone is called facultative water reabsorption.
Antidiuretic hormone is released more or less continuosly unless the blood solute concentration drops too low. Released of antidiuretic hormone is enhanced by any event that raises plasma osmolality above 300mOsm, such as sweating or diarrhea, or by greatly reduced blood volume or blood pressure. Although release of antidiuretic hormone is the "signal" to produce concentrated urine that opens the door of water reabsorption (through aquaporins), the kidney's ability to respond this signal depends on the high medullary osmotic gradient,
Dilute urine
Tubular filtrate is diluted as it travels through the ascending limb of the loop of Henle, so all the kidney needs to do secrete dilute (hypo-osmotic) urine is allow the filtrate to continue on its way into the renal pelvis. When ADH (Antidiuretic hormone) is not being released by the posterior pituitary, that is exactly what happens, The collecting ducts remain essentially impermeable to water due to absence of aquaporins in their luminal cell membranes, and no further water reabsorption occurs. Moreover, as noted, Na+ and selected other ions can be removed from the filtrate by distal convoluted tubule and collecting duct cells so that urine becomes even more dilute before entering renal pelvis. The osmolality of urine can plunge as low as 50 mOsm, about one-sixth the concentration of lomerular filtrate or blood plasma.
Formation of dilute or concentrated urine
The kidneys go to a great deal of trouble to create the medullary osmotic gradient. What is its purpose? Without this gradient, you would not be able to raise the concentration of urine above 300 mOsm, the osmolality of interstitial fluid. As a result, you would not be able to excrete excess solutes to lower your body osmolality.
Controlling the reabsorption of water from filtrate in the collecting ducts in order to adjust the body's osmolality is the job of antidiuretic hormone (ADH). ADH inhibits diuresis, or urine output. It accomplishes this via a second messenger system using cyclic AMP that causes insertion of aquaporins into the luminal membrane of the principal cells of the collecting ducts. The amount of the ADH determines the number of aquaporins in the collecting ducts and so the amount of water that is reabsorbed there.
The countercurrent exchanger
The vasa recta function as counter current exchangers, maintaining the osmotic gradient established by the cycling of salt while delivering blood cells in the area and removing reabsorbed water and solutes. These vessels receive only about 10% of the renal blood supply, making blood flow through the vasa recta sluggish. Moreover, they are freely permeable to water and NaCl, allowing blood to make passive exchanges with the surrounding interstitial fluid. Consequently, as the blood flows into the medullary depths, it loses water and gains salt (becomes hypertonic). Then, as it emerges from the medulla into the cortex, the process is reversed. It picks up water and loses salt. The water picked up by the ascending vasa recta includes not only water lost from the descending vasa recta, but also water reabsorbed from the loop of Henle and collecting duct. As a result, the volume of the blood at the end of the vasa recta is greater than at beginning.
Because blood leaving and reentering the cortex via the vasa recta has nearly the same solute concentration, the vessels of the vasa recta act as countercurrent exchangers. This system does not create the medullary gradient, but it protects it by preventing rapid removal of salt from the medullary interstitial space, and by removing reabsorbed water,
Urea recycling and the medullary osmotic gradient
In addition to Na+, urea forms an important part of the medullary osmotic gradient. Urea enters the filtrate by facilitated diffusion in the ascending thin limb of the loop of Henle. As the filtrate moves on, water is usually reabsorbed in the cortical collecting duct, leaving urea behind. When filtrate reaches the collecting duct in the deep medullary region, urea, now highly concentrated, is transported by facilitated diffussion out of the tubule into the interstitial fluid of the medulla, forming a pool of urea contributes substantially to the high osmolality in the medulla.
Anti diuretic hormone (ADH), which stimulates excretion of highly concentrated urine, enhances urea transport in the medullary collecting duct. When ADH is present, urea recycling is enhanced, the medullary osmotic gradient is enhanced, and more concentrated urine can be formed.
The countercurrent multiplier
First, we will follow filtrate processing through the loop of Henle, to see how the loop functions as a countercurrent multiplier to establish the osmotic gradient. The countercurrent multiplier functions because of two factors:
- The descending limb of the loop of Henle is relatively impermeable to solute and freely permeable to water. Water passes osmotically out the filtrate all along this limb because the osmolality of the medullary interstitial fluid increases all along the descending limb. The filtrate osmolality reaches its highest point (1200 mOsm) at that bend of the loop.
- The ascending limb is permeable to solutes, but not to water. As the filtrate rounds the bend into the ascending limb, the tubule permeability changes, becoming impermeable to water and selectively permeable to salt. The Na+ and Cl- concentration in the filtrate entering the ascending limb is very high (and interstitial fluid concentrations of these two ions are lower). Na+ and Cl- reabsorption in the ascending limb is both passive (mostly in the thin segment) and active (via Na+K+2Cl- cotransporter in the thick segment). As Na+ and Cl- are extruded from the filtrate into the medullary interstitial fluid, they contribute to the high osmolality there. Because it losses salt but not water, the filtrate in the ascending limb becomes increasingly dilute. Finally, at 100 mOsm at the distal convoluted tube, it is hypoosmotic , or hypotonic, to blood plasma and cortical interstitial fluids.
There is a constant diffence in filtrate concentrations (200 mOsm) between the two limbs of the loop of Henle, and between the ascending limb and the interstitial fluid. This difference reflects the power of the ascending limb's NaCl pumps, which are just powerful enough to create a 200 mOsm difference between the inside and outside of the ascending limb. A 200 mOsm gradient by itself would not be enough to allow excretion of very concentrated urine. The beauty of this system lies in the fact that, because of countercurrent flow, the loop of Henle is able to "multiply" these small changes in solute concentrations into a gradient change along the vertical length of the loop (both inside and outside) that is closer to 900 mOsm (1200 mOsm - 300 mOsm).
Although the two limbs of Henle are not in direct contact with each other, they are close enough to influence each other's exchanges with the interstitial fluid they share. Water diffusing out of the descending limb leaves behind an increasingly "salty" filtrate that the ascending limb then uses to raise the osmolality of the medullary interstitial fluid, Furthermore, the more NaCl the ascending limb extrudes, the more water diffuses out of the descending limb and the saltier the filtrate in the descending limb becomes. This establishes a positive feedback cycle that produces the high osmolality of the fluids in the descending limb and interstitial fluid.
Regulation of urine concentration and volume
One crucial renal function is to keep the solute concentration of body fluids constant. we use osmolality to measure the amount of solutes in the body fluids. A solution's osmolality is the number of solute particles dissolved in 1 kg of water and reflects the solution's ability to cause osmosis. For any solution interfacing with a selectively permeable membrane, this ability, called osmotic activity, is determined only by the number of solute particles unable to pass through the membrane (called non penetrating solute particles) and is independent of their type. For example, 10 sodium ions have the same osmotic activity as 10 glucose molecules or 10 amino acids in the same volume of water.
Because 1 osmol (equivalent to 1 mole of particles) is a fairly large unit, the milliosmol (mOsm) equal to 0.001 osmol, is generally used.
The kidneys keep the solute load of body fluids constant at about 300 mOsm, the osmotic concentration of blood plasma, by regulating urine concentration and volume. The kidneys accomplish this feat using countercurrent mechanism. In the kidneys, the term countercurrents means that fluid flows in opposite directions through adjacent segments of the same tube connected by a hairpin turn. These countercurrent mechanism are (1)the interaction between the flow of filtrate through the ascending and decending limbs of the long loops of Henle of juxtamedullary nephrons (the counter current multiplier), and (2)the flow of blood through the ascending and decending portions of the vasa recta blood vessels (the countercurrent exchanger). These countercurrent mechanisms establish and maintain an osmotic gradient extending from the cortex through the depths of the medulla. This gradient allow the kidneys to vary urine concentration dramatically.
The osmolality of the filtrate entering the proximal convoluted tubule is identical to that of plasma, about 300 mOsm, Because of proximal convoluted tubule reabsorption of water and solutes, the filtrate is still isoosmotic with plasma by the time it reaches the decending limb of the loop of Henle. However, its osmolality increases from 300 to about 1200 mOsm in the deepest part of medulla.
How does this increase in concentration occur? The answer lies in the unique workings of the long loop of the juxtamedullary nephrons, and the vasa recta.
Tubular secretion
The failure of tubule cells to reabsorb some solutes is an important way of clearing plasma of unwanted substances. Another way is tubular secretion-essentially, reabsorption reverse. Substances such as H+, K+,NH4+, creatinin and certain organic acids either move into the filtrate from the peritubular capillaries through the tubule cells or are synthesized in the tubule cells and secreted. As a result, the urine eventually excreted contains both filtered and secreted substances. With one major exception (K+), the proximal convoluted tubule is the main site of secretion, but the cortical parts of the collecting ducts are also active.
Tubular secretion is important for:
Disposing of substances, such as certain drugs and metabolites, that are tightly bound to plasma protein. Because plasma proteins are generally not filtered, the substances they bind are not filtered and so must secreted.
Eliminating undesirable substances or end products that have been reabsorbed by passive processes. Urea and uric acid, two nitrogenous wastes, are both handled in this way. Urea handling in the nephron is complicated, but the net effect is that 40-50% of the urea in the filtrate is excreted.
Ridding the body excess K+. Because virtually all K+ present in the filtrate is reabsorbed in the proximal convoluted tubule and ascending loop of Henle, nearly all K+ in urine is from aldosteron driven active tubular secretion into the late distal convoluted tubule and collecting ducts.
controlling blood pH. When blood pH drops toward acidic end of its homestatic range, the renal tubule cells actively secrete more H+ into the filtrate and retain and generate more HCO3- (a base). As a result, the blood pH rises and the urine drains off the excess H+. Conversely, When blood pH approaches the alkaline end of its range, Cl- is reabsorbed instead of HCO3-, which is allowed to leave the body in urine.
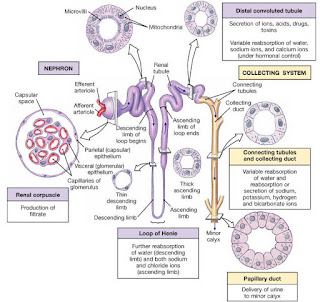
Reabsorptive capabilities of the renal tubules and collecting ducts part 1
Proximal convoluted tubule
The entire renal tubule is involved in reabsorption to some degree, but the proximal convoluted tubule cells are by far the most active "reabsorbers" and the events just described occur mainly in this tubular segement. Normally, the proximal convoluted tubule reabsorbs all of the glucose, lactate and amino acids in the filtrate and 65% of the Na+ and water. Additionally, 80% of the filtered bicarbonate (HCO3-), 60% of Cl- and about 55% of the K+ are reclaimed in the proximal convoluted tubule. The bulk of the reabsorption of electrolytes is accomplished by the time the filtrate reaches the loop of Henle. Nearly all of the uric acid and about half of the urea are reabsorbed in the proximal tubule, but both are later secreted back into the filtrate.
Loop of Henle
Beyond the proximal convoluted tubule, the permeability of the tubule epithelium changes dramatically. Here, for the first time, water reabsorption is not coupled to solute reabsorption. Water can leave the descending limb of the loop of Henle but not the ascending limb, where aquaporins are scarce or absent in the tubule membrane. These permeability differences play a vital role in the kidneys ability to form dilute and concentrated urine.
The rule for water is that it leaves the descending (but not the ascending) limb of Henle's loop, and the opposite is true for solutes. Virtually no solute reabsorption occurs in the descending limb. but both active and passive reabsorption of solute occurs in the ascending limb. In the thin portion of the ascending limb, Na+ moves passively down the concentration gradient created by water reabsorption. A Na+K+2Cl- symporter is the main means of Na+ entry at the luminal surface in the thick portion of the ascending limb . A Na+K+ATPase operates at the basolateral membrane to create the ionic gradient that drives the symporters. The thick asceding limb also has Na+H+ antiporters. In addition, some of 50% of Na+ passes via the paracellular route in this region.
Tubular reabsorption part 3
Reabsorption of nutrients, water and ions
The reabsorption of Na+ by primary active transport provides the energy and the means for reabsorbing almost every other substance, including water. Substances reabsorbed by secondary active transport (the "push" comes from the gradient created by Na+K+ pumping at the basolateral membrane) include glucose, amino acids, lactate and vitamins. In nearly all these cases, a luminal carrier moves Na+ down its concentration gradient as it cotransports (symports) another solute. Cotransported solutes diffuse (via different transport proteins) across the basolateral membrane before moving into the peritubular capillaries. Although there is some overlap of carriers, the transport systems for the various solutes are quite specific and limited.
There is a transport maximum (Tm) for nearly every substance that is reabsorbed using a transport protein in the membrane. The Tm (reported in mg/min) reflects the number of transport protein in the renal tubules available to ferry each particular substance. In general, there are plenty of transporters and therefore high Tm values for substances such as glucose that need to be retained, and few or no transporters for substances of no use to the body.
When the transportes are saturated,-that is, all bound to the substance they transport-the excess is excreted in urine. This is what happens in individuals who become hyperglycemic because of uncontrolled diabetes mellitus. As plasma levels of glucose approach and exceed 180 mg/dl, the glucose Tm is exceeded and large amounts of glucose may be lost in the urine even though the renal tubules are still functioning normally.
In passive tubular reabsorption, which encompasses osmosis, diffussion, and facilitated diffusion, substances move down their electrochemical gradients without the use of ATP. The movement of Na+ and other solutes establishes a strong osmotic gradient, and water moves by osmosis into the peritubular capillaries, a process aided by transmembrane proteins called aquaporins that form water channels across cell membrane. In continuosly water-permeable regions, aquaporins are constant components of the tubule cell membranes. Because these channels are always present, the body is "obliged" to absorb water in the proximal nepron regardless of its state of over or underhydration. This water flow is referred to as obligatory water reabsorption. Aquaporins are virtually absent in the luminal membranes of the collecting duct unless antidiuretic hormone (ADH) is present.
As water leaves the tubules, the concentration of solutes in the filtrate increases and, if able, they to begin to follow their concentration gradients into the peritubular capillaries. This phenomenon of solutes following solvents explains the passive reabsorption of a number of solutes present in the filtrate, such as lipid-soluble substances, certain ions and some urea. It also explains in part why lipid-soluble drugs and enviromental toxins are difficult to excrete, since lipid-soluble compounds can generally pass through membranes, they will follow their concentration gradients and be reabsorbed, even if this is "not desirable".
As they move through the tubule cells into the peritubular capillary blood, Na+ ions also establish an electrical gradient that favors passive reabsorption of anions (primarily Cl-) to restore electrical neutrality in the filtrate and plasma.
Any plasma proteins that squeeze through the filtration membrane are removed from the filtrate in the proximal tubule by endocytosis and digested to their amino acids, which are moved into the peritubular blood.
Tubular reabsorption part 2
SODIUM REABSORPTION (Na+)
Sodium ions (Na+) are the single most abundant cation in the filtrate, and about 80% of the energy is used for active transport is devoted to their reabsorption. Sodium reabsorption is almost always active and via the transcellular route.
In general, two basic processes that promote active Na+ reabsorption occur in each tubule segment.
First, Na+ is actively transported out of the tubule cell by primary active transport-a Na+K+ ATPase pump present in the basolateral membrane. From there, Na+ is swept along by the bulk flow of the water into adjacent peritubular capillaries is rapid because the blood there has low hydrostatic pressure and high osmotic pressure (remember, most protein remain in the blood instead of being filtered out into the tubule).
Second, active pumping of Na+ from the tubule cells result in a strong electrochemical gradient that favors its passive entry at the luminal face via secondary active transport carries or via facilitated diffusion through channels. This occurs because (1)the pump maintains the intracellular Na+ concentrations at low levels (2)the K+ pumped into the tubule cells almost immediately diffuses out into the interstitial fluid via leakage channels, leaving the interior of the tubule cell with a net negative charge.
Because each tubule segment plays a slightly different role in reabsorption, the precise mechanism by which Na+ is reabsorbed at the luminal membrane varies.
Tubular reabsorption part 1
Total plasma volume filters into the renal tubules about every 22 minutes, so all plasma would be drained away as urine in less than 30 minutes were it not for the fact that most of the tubule contents are quickly reclaimed and returned to the blood. This reclamation process, called tubular reabsorption, is a selective transepithelial process that begins as soon as the filtrate enters the proximal tubules. To reach the blood, reabsorbed substances follow either either the transcellular or paracellular route. In the transcelullar route, transported transported substances move through the luminal membrane, the cytosol, and the basolateral membrane of the tubule cell and then the endothelium of the peritubular capillaries. Movement of substances in the paracellular route between the tubule cells is limited because these cells are connected by tight junctions. In the proximal nephrons, however, these tight junctions are "leaky" and allow some important ions (Ca2+, Mg2+, K+, and some Na+) through the paracellular route.
Given healthy kidneys, virtually all organic nutrients such as glucose and amino acids are completely reabsorbed to maintain or restore normal plasma concentrations. On the other hand, the reabsorbtion of water and many ions is continuosly regulated and adjusted in response to hormonal signals. Depending of the substances transported, the reabsortion process may be passive (no ATP required) or active (at least one of its steps is driven by ATP directly or indirectly)
Wednesday, July 11, 2018
Glomerular filtration part 2C
Other factors affecting Glomerular filtration rate
Renal cells produce a battery of chemicals, many of which act as paracrines (local signaling molecules) :
- Prostaglandin E2 (PGE2) : The vasodilatory paracrine PGE2, counteracts vasoconstriction by norepinephrine and angiotensin II within the kidney. The adaptive value of these opposing actions is to prevent renal damage while responding to body demands to increase peripheral resistance
- Intrarenal angiotensin II : Although we usually think of angiotensin II as hormone, the kidney makes its own, locally acting angiotensin II that reinforces the effects of hormonal angiotensin II, It also dampens the resulting renal vasoconstriction by causing PGE2 release.
- Adenosine : Adenosine can be released as such or produced extracellularly from ATP released by macula densa cells. Although it functions as avasodilator systemically, adenosine constricts the renal vasculature
Abnormally low urine output (less than 50 ml/day), called anuria, may indicate that glomerular blood pressure is too low to cause filtration. However, renal failure and anuria can result from situations in which the nephrons cease to functions for a variety of other reason, including acute nephritis, transfusion reactions and crush injuries.
Glomerular filtration part 2B
EXTRINSIC CONTROLS (NEURAL AND HORMONAL MECHANISM)
The purpose of the extrinsic controls regulating the glomerular filtration rate is to maintain systemic blood pressure - sometimes to the detriment of the kidneys.
- Symphatetic nervous system controls
Norepinephrine released by symphatetic nerve fibers (and epinephrine released by the adrenal medulla) acts on alpha-adrenergic receptors on vascular smooth muscle, strongly constricting afferent arterioles, thereby inhibiting filtrate formation. This, in turn, indirectly trips the renin-angiotensin mechanism by stimulating the macula densa cells. The sympathetic nervous system also directly stimulates the granular cells to release renin.
- RENIN-ANGIOTENSIN MECHANISM
Angiotensin II acts in five ways to stabilize systemic blood pressure and extracellular fluid volume. (1) As a potent vasoconstrictor , angiotensin II activates smooth muscle of arterioles throughout the body, raising mean arterial blood pressure. (2) Angiotensin II stimulates reabsorption of sodium, both directly by acting on renal tubules and indirectly by triggering the release of aldosterone from the adrenal cortex. Because water follows sodium osmotically, blood volume and blood pressure rise. (3) Angiotensin II stimulates the hypothalamus to release anti diuretic hormone and activates the hypothalamic thirst center, both of which increase blood volume. (4) Angiotensin II also increases fluid reabsorption by decreasing peritubular capillary hydrostatic pressure. This pressure drop occurs because the efferent arterioles constrict, and the downstream drop in hydrostatic pressure allows more fluid to move back into the peritubullar capillary bed. (5) Angiotensin II targets the glomerular mesangial cells, causing them to contract and reduce the glomerulus filtration rate by decreasing the total surface area of glomerular capillaries available for filtration.
While this seems daunting list at first, it will help that all of the effects of angiotensin II are aimed at restoring blood volume and blood pressure. Of angiotensin II's many effects, the first two are the most important.
Several factors acting independently or collectively can trigger renin release:
- Reduced stretch of the granular cells. A drop in mean systemic blood pressure below 80 mmHg (as might be due to hemorrhage, dehydration, etc) reduces the stretch of the granular cells and stimulates them to release more renin
- Stimulation of the granular cells by input from activated macula densa cells. When macula densa cells sense low NaCl concentration (slowly moving filtrate), they signal the granular cells to release renin. This signal may decrease release of ATP (also thought to be the tubuloglomerular feedback messenger), increased release of the prostaglandin PGE2, or both
- Direct stimulation of granular cells via β1-adrenergic receptors by renal symphatetic nerves.
Glomerular filtration part 2A
Regulation of glomerular filtration
Glomerular filtration rate is regulated by both intrinsic and extrinsic controls. These two types of controls serve two different (and sometimes opposing) needs. The kidneys need a relatively constant glomerular filtration rate in order to do their job and maintain extracellular homeostasis. On the other hand,the body as the whole needs a constant blood pressure, and therefore a constant blood volume.
Intrinsic controls (renal autoregulation) act locally within the kidney to maintain glomerular filtration rate, while extrinsic controls by the nervous and endocrine systems maintain blood pressure. In extreme changes of blood pressure (mean arterial pressure less than 80 or greater than 180 mmHg), extrinsic control take precedence over intrinsic controls.
INTRINSIC CONTROLS (RENAL AUTOREGULATION)
By adjusting its own resistance to blood flow, a process called renal autoregulation, the kidney can maintain a nearly constant glomerular filtration rate despite fluctuations in systemic arterial pressure. Renal autoregulation entails two types of controls :
- Myogenic mechanism
- Tubuloglomerular feedback mechanism
On the other hand, when macula densa cells are exposed to slowly flowing filtrate with its low NaCl concentration, ATP release is inhibited, causing vasodilatation of the afferent arterioles. This allows more blood to flow into the glomerulus, thus increasing net filtration presure and glomerulus filtration rate.
Autoregulatory mechanism maintain a relatively constant glomerulus filtration rate over an arterial pressure range from about 80 to 180 mmHg. Consequently, our normal day to day activity (such as exercise, sleep or changes in posture) do not cause large changes in water and solute excretion. However, the intrinsic controls cannot handle extremely low systemic blood pressure, such as might result from serious hemorrhage (hypovolemic shock). Once the mean arterial pressure drops below 80 mmHg, autoregulation ceases.
Subscribe to:
Posts (Atom)