Thursday, August 30, 2018
Water purity and quality
Quality standards for water and concentrate used to produce dialysate are well established. The European Renal Association standard stipulates that microbiological contamination of the delivered water should comply with the recomendations of the European Pharmacopoeia bacterial count of - 100 colony-forming unit/ml and endotoxin content of -0.25 endotoxin unit/ml. All convective methods require ultrapure water (endotoxin level < 0.03 endotoxin unit/ml). Regular monitoring of water quality, preferably monthly, should be performed. Ultrapure dialysate is defined as a bacterial count <0.1 colony-forming unit/ml and an endotoxin level <0.03 endotoxin unit/ml. This endotoxin level is the sensitivity treshold for the simplest of the limulus amoebocyte lysate assays.
Bacterial products, such as endotoxins, fragmen of endotoxin, peptidoglycans, and fragments of bacterial DNA, can cross the pores into the blood stream. These are all potent inducers of cytokynes and stimulators of the acute phase response contributing to chronic inflammation. Recently, circulating endotoxins have been shown to have potential impact on survival in patients undergoing peritoneal dialysis or hemodialysis. The introduction of ultrafiltered dialysate was associated with a significant reduction in plasma ß2-microglobulin concentration and a significant improvement in nutritional status, assessed by plasma albumin concentration and creatinine generation rate as marker of muscle mass,
Dialysate composition - Buffering
Bicarbonate dialysate is now the fluid of choice. Bicarbonate is the primary buffer that traditionally has been prescribed in the range of 33-38 mmol/l. The concentration of acetate used is variable and contributes to the total buffering, with a range of 4-8 mmol/l, whereas 5-6 mmol/l is most widely used and appears to be safe, providing these sources of bicarbonate concentration are considered when the dialysate prescription is written. The aim of intradialytic buffering is to avoid post dialysis alkalosis and acidosis before the next session. Net acidosis may lead a catabolic state, insulin resistance and bone loss. Net alkalosis may lead to reduced cerebral flow, cramps and fatigue. Recent observational data have demonstrated that high dialysate bicarbonate (>35 mmol/l) may be associated with the adverse outcomes. Modeling dialysate bicarbonate is of uncertain benefit, but lower bicarbonate may assist patients with intradialytic hypotension.
Dialysate composition - GLUCOSE
Hemodialysate solutions often contain high concentrations of glucose (up to 200 mg/dl). The historical reasons for the addition of glucose to the dialysate including following:
- enhancing ultrafiltration
- minimization of nutritional (caloric) loses during dialysis
Dialysate composition - CALCIUM
Lower dialysate calcium (1.25-1.5 mmol/l) may reduce the risk of hypercalcemia, but may lead to negative calcium balance, hyper parathryroidism, and intradialytic hypotension. Increased attention has been paid recently to integrating choice of dialysate calcium level into the understanding of calcium balance, which is determined by diffusive intradialytic fluxes, the dietary calcium content, and the administration of calcium-containing phosphat binders, as well as by dosage of vitamin D analogs. If hypokalemia is coexistent, then critical QTc prolongation may occur.
Higher dialysate calciums level (1.5-1.75 mmol/l) may improve hemodynamic stability during dialysis, but may also increased the risk of hypercalcemia and vascular calcification.
Given that dialysate calcium is only one component in the total calcium balance, dietary calcium, calcium-containing phosphat binders, and the use of vitamin D or its analogs also need to be considered when dialysate calcium is prescribed
Dialysate composition - POTASSIUM
Hypokalemia and hyperkalemia may lead to potentially life threatening cardiac arrhytmias. The usual dialysate potassium level is 2 mmol/l. Low dialysate concentration, particularly those of 0 or 1 mmol/l, Should be avoided. If used, extreme caution should be exercised because the rapid decline in plasma potassium concentration, which occurs in the early stages of a dialysis treatment, is arrhythmogenic. Haemodialysis is associated with the markers of cardiac electrophysiologic abberancy, particularly in patients with underlying cardiovascular disease, and those markers are amplified by a low potassium bath. Reducing the blood-to-bath potassium gradient during dialysis mitigates the dialysis-associated electrophysiologic effects. However, the cardiacelectrophysiologic markers appear to add little or nothing to the sudden death risk assessment and so are of dubious predictive value. The risk of intradialytic hypotension inversely correlates to the potassium concentration in the dialysate. The use of 1 mmol/l potassium dialysate in a chronic hemodialysis setting has been associated with an increased incidence of cardiac arrest. Similar to sodium, potassium profiling has been suggested, but the evidence is weak.
Dialysate composition - SODIUM part 3
Individualized therapy according to the set point, which implies alignment of dialysate and serum sodium, has been advocated. Sodium profiling could be considered persistently symptomatic in patients because of intradialytic hypotension or disequilibrium symptoms. However, evidence supporting these approaches is weak. On first principles, the aim of dialysate treatment should be to removed quantity of sodium accumulated since the last session, but feasible and accurate methods to achieve that aim are currently unavailable. Alignment of patient serum and dialysate sodium concentrations assists in achieving this goal. Avoiding sodium loading in hemodialysis patients is a cornerstone for blood pressure and fluid status management.
Dialysate composition - SODIUM part 2
The use of dialysate sodium that is markedly lower compared with the patient's serum sodium results in rapid reduction in the plasma osmolality and intravascular volume, leading to disequilibrium symptoms and hypotension, however, when used at smaller gradients, sodium will not flux into the patient and, post dialysis thirst is prevented without undesireable hemodynamic events. Dialysate sodium higher than serum sodium may help to maintain blood pressure with ultrafiltration, but leads to post-dialytic thirst, fluid induced weigh gain and hypertension. Creating a positive intradialytic sodium balance is effective in acutely reducing the incidence of intradialytic symptoms, but it also sustains a vicious cycle hampering the attainment of dry weight and pre disposes the patient to an increased risk of intra dialytic complications during the following dialysis session. An isonatric hemodialysis may have a beneficial effect on blood pressure and dialysis tolerance. A biofeedback system using hemodialysis filtrations regeneration of ultrafiltrate has been specially developed with an isonatric mode maintaining an equal serum sodium concentration between the start and the end of the dialysis session, combined with ultrafiltration and conductivity profiles.
Dialysate composition - SODIUM part 1
SODIUM
Sodium has a critical role in the regulation of weight, extracellular fluid volume, blood pressure and thirst. The sodium concentration in plasma water exceeds that in plasma by about 10 mmol/l, suggesting a large gradient driving sodium into the dialysate. However, most of this apparent gradient is eliminated by the effect of negatively charged plasma proteins that reduce the diffusible sodium. Hence, the plasma sodium concentrations is generally used instead of plasma "water" sodium as the relevant concentrations. This plasma sodium concentration may be a bit less, on average,compared with the diffusible, ionized sodium, and that the correlation between the diffusible sodium and the plasma sodium levels may differ a bit in individual patients. Sodium removal during haemodialysis can occur through convection or diffusion. Current prescribing practices for chronic intermittent hemodialysis rely primarily on convective losses (∼78%) and less on diffusive lossess (∼22%). Hypothetically, a regular removal of 1L of ultrafiltered plasma water, considering a theoritical isotonic sodium concentrations of 140 mmol/l in the ultrafiltrate, would be responsible for a removal of 140 mmol of sodium, equivalent to 8 g of sodium chloride ingestion in each interdialytic day. The usual dialysate sodium level is between 135 and 145 mmol/l. In general, a high-sodium dialysate would be above 141 mml/l, whereas below 137 mmol/l would be regarded as low sodium dialysate.
Dialysate
In many dialysis units worldwide, dialysate is prepared centrally and to standardized prescription, which can be modified to an extent by most dialysis machines. More recently, with an older and more complex group of patients on long-term hemodialysis, in an attemp to reduce intradialytic and interdialytic complications and improve long-term outcomes, individualized prescriptions have been developed, evaluated and used. In general, accross all dialysate components, homeostasis is achieved best with "middle-range" prescriptions
Tuesday, August 28, 2018
Safety and ease of use
In addition to concerns about the accuracy of the displayed blood flow rate, the effective blood flow rate may be lower than the pumped blood flow rate because of access recirculation. Access recirculation occurs when the blood flow entering the access from the systemic circulation is insufficient to provide the chosen extracorporeal blood flow rate. When that occurs, some of blood returning to the acces from the dialyzer is immediately recycled back to the dialyzer. Traditionally, access recirculation was determined from blood urea concentrations in the extracorporeal circuit and systemic circulation. This methode have several drawbacks, it may be confounded by cardiopulmonary recirculation and it may require several days to obtain the necessary urea concentrations from a clinical laboratory. An alternative approach to monitoring access performance is to measure the access blood flow rate. Access blood flow rate can be measured using indicator dilution methods. This technique provides a reliable estimate of access blood flow rate but requires additional equipment. Access blood flow rate can also be calculated from clearances measured with the blood lines in the normal configuration and in a reverse configuration. This method has the advantage that several dialysis machines now allow the automated non-invasive measurements of ionic clearance based on dialysate conductivity measurements.
Efficiency of dialysis part 2
In addition to concerns about the accuracy of the displayed blood flow rate, the effective blood flow rate may be lower than the pumped blood flow rate because of access recirculation. Access recirculation occurs when the blood flow entering the access from the systemic circulation is insufficient to provide the chosen extracorporeal blood flow rate. When that occurs, some of blood returning to the acces from the dialyzer is immediately recycled back to the dialyzer. Traditionally, access recirculation was determined from blood urea concentrations in the extracorporeal circuit and systemic circulation. This methode have several drawbacks, it may be confounded by cardiopulmonary recirculation and it may require several days to obtain the necessary urea concentrations from a clinical laboratory. An alternative approach to monitoring access performance is to measure the access blood flow rate. Access blood flow rate can be measured using indicator dilution methods. This technique provides a reliable estimate of access blood flow rate but requires additional equipment. Access blood flow rate can also be calculated from clearances measured with the blood lines in the normal configuration and in a reverse configuration. This method has the advantage that several dialysis machines now allow the automated non-invasive measurements of ionic clearance based on dialysate conductivity measurements.
Efficiency of dialysis part 1
Small solute clearance is mostly dependent on the blood and dialysate flow rates. It has long been known that the flow rate produced by the type of peristaltic pump used in the blood circuit is influenced by the inlet pressure to the pump. As the pressure decreases, the pump segment of the tubing exhibits incomplete elastic recoil resulting in a decrease in the cross-sectional area of the pump segment. Since the blood flow rate displayed by dialysis machines is determined by the number of pump revolutions and stroke volume, which is the product of the length and cross sectional area of the pump segment, actual blood flow rates may be substantially lower than displayed blood flow rates. This discrepancy can lead to underdelivery of the dialysis prescription. Two approaches have been taken to address this problem. Some dialysis machines now display blood flow rates corrected for the pressure at the pump inlet using a software algorhythm. A new blood tubing set that is less pressure sensitive has been introduced. The pump segment of this tubing set is more elastic and undergoes more complete recoil than traditional tubing set.
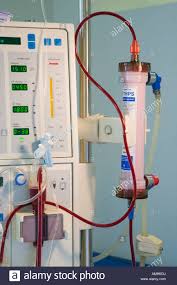
Dialysis machine
Dialysis machine have three basic functions:
- circulation of blood from the patient's access through the dialyzer and back to the access using a blood pump and a disposable tubing set
- preparation of dialysate from purified water and one or more concentrates and circulation of that dialysate through the dialyzer using a system that also controls the rate of fluid removal
- monitoring for any loss of integrity in either the blood or dialysate circuit or any excursion of an operating parameter outside a predefined range
Clearance of large solute part 2
Newer high-flux dialyzer offer increased clearances for large molecular weight solutes, particularly when used in a therapy with convection, there may be a limit to the benefits that accrue from their use because of limitations imposed by intra-body mass transfer.When mass transfer in the body becomes limiting, changing treatment time and frequency will be more important than increasing clearance.
A recent development has been the introduction of protein-leaking membranes for hemodialysis. These membranes allow the passage of larger molecules than do traditional high-flux membranes at the cost of some albumin loss. Preliminary clinical studies suggest that use of protein leaking membranes may aid in anemia correction, reduce hyperhomocysteinemia and reduce plasma concentrations of glycosylated and oxidized proteins.
Clearance of large solute part 1
It is generally accepted that the uremic toxins cover a wide molecular weight range. Removal of higher molecular weight solutes necessitates the use of high-flux dialyzers, since low-flux dialyzer have essentially no capacity to remove solutes the size of small proteins. The most common high-flux membranes have some form of polysulfone as their major component. However, there are differences in the composition and structure of various high-flux "polysulfone" membranes, which may be associated with significant differences in performance. It is not yet clear if the differences in performance arise from differences in the polymer composition of the membrane or from differences in membrane morphology.
Removal of large molecular weight solutes by high-flux membranes is enhanced when the treatment includes convection, either as the sole means of solute removal (hemofiltration) or in combination with diffusion (haemodiafiltration). Convection provides more removal of large molecules than diffusion because the sieving coefficient decreases more gradually with increasing molecular size than does the mass transfer coefficient. Convective therapies are performed most efficiently when replacement solution is prepared on-line. Equipment to perform on-line therapies is widely available. In the absence of purpose-design equipment, the advantages of convection may be partially realized by maximizing internal filtration and back-filtration in the dialyzer. Internal filtration and back filtration occur when membranes of high water permeability are used with a volume control system, because the low transmembrane pressure required for fluid removal and the counter-current flow of blood and dialysate in a dialyzer create local pressure gradients favoring filtration from blood to dialysate at the blood inlet end of the dialyzer. Internal filtration can be increased by using smaller diameters fibers to increase the pressure at the blood inlet.
Clearance of small solutes
For small solutes, membrane resistance is now at a level where fluid boundary layers are significant contributors to the mass transfer coefficient. There has been little effort at technological innovation to decrease boundary layer resistance on the blood side of the membrane. In contrast, several design changes have been introduced to decrease dialysate side resistance. These changes, aimed at improving the flow ditribution of dialysate through the fiber bundle in the response to the observations that the mass transfer coefficient and the membrane surface area increases as the dialysate flow rate increases, include optimization of fiber packing density, use of spacer yarns, fiber undulations, and new flow distributor design. These changes have improved clearance of small solutes. How ever , the increases are small, generally less than 10%-reflecting the dominant role that blood flow rate plays in the determining the clearance of small solutes.
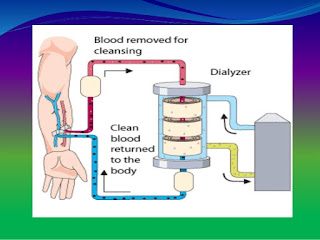
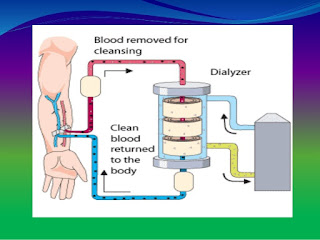
Dialyzer
All dializers now in clinical use are of the hollow fiber type with membranes of cellulose, modified cellulose or synthetic polymers. The function of a dialyzer is to return the internal milieu to a more normal state through water removal and solute exchange between blood and dialysate. Water removal depends on the hydraulic permeability of the membrane. Automated ultrafiltration control systems are a standard feature of current dialysis machine and have eliminated the potential for excessive, uncontrolled ultrafiltration. Thus, the hidraulic permeability of the membrane is no longer an issue in the clinical use of modern dialyzers. With hydraulic permeability no longer a concern, membrane manufacturers have been free to engineer membranes to maximise solute exchange.
Solute transfers in hemodialysis is determined by the diffusive and convective permeability of the membrane-defined by the mass transfer coefficient and the sieving coefficient, respectively-the membrane surface area, and the blood and dialysate flow rates. The mass transfer coefficient is a composite parameter that incorporates the resistances to mass transfer associated with the membrane and the fluid boundary layers on the blood and dialysate sides of the membrane
What if I do not want to reuse my dialyzer?
Many dializer centers will provide you with a new dialyzer for each treatment if you ask for one. However, the dialyzer may not be a high-flux dialyzer, and you may have to spend extra time on the machine to get enough treatment. Ask your dialysis care team about your center's policy on reuse.
How can I be sure I am getting the right amount of treatment from my reused dialyzer?
To help ensure that you are getting the most from your dialysis treatment, ask your dialysis care team about your monthly urea reduction ratio. If your numbers are too low, you may not be getting enough dialysis. A dialyzer that is not working well is one reason why this may occur.
How can I be sure that I am not using someone else's dialyzer?
Before each treatment it is important that you and your nurse or technician check the label on your reused dialyzer. The label should be clearly marked with your name.
How should my dialyzer look before treatment?
Your dializer should look clean. If your dialyzer looks clotted or dirty, speak to your dialysis care team before starting your treatment. Always check the appearance of your dialyzer before each treatment. Make sure the dialyzer:
- looks clean
- has no more than a few clotted fibers
- has clean tops and bottoms that are free of all but small clots
- is not leaking
- is capped on all openings or ports
- is clearly labeled with your name
How many times can I safely reuse my dialyzer?
There is no set number of times that is considered safe for dialyzer reuse. As long as the total cell volume test shows that the dialyzer is working well, and the dialyzer looks clean, it should be safe for you to reuse your dialyzer.
Dialyzer test
Total Cell Volume
Total cell volume is a test to see how well your dialyzer is working. According to the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (NKF's KDOQI), total cell volume should always be at least 80% of what it was first.
Urea Reduction Ratio
Urea reduction test should be at least 65%, if the right amount of dialysis is delivered to the patient, A dialyzer that is not working well is one possible reason why your numbers may be too low.
Thursday, August 23, 2018
How do I know that my dialyzer is working well?
The dialyzer to be reused should be tested in a special way before its first use and again every time it is reused to make sure it is working effectively. A dialyzer that is not working well may be one of the possible causes of:
- loss of appetite
- loss of body weight
- nausea and vomitting
- changes in your monthly lab tests such as BUN, creatinine and albumin
What do the reuse guidelines say?
Some of the important things the guidelines say are:
- Dialyzers should be labelled carefully and always used for the same patient
- Dialyzers should be tested after rinsing to make sure all disinfectants have been removed
- Patients should be checked for any bad reactions caused by reuse
- Dialyzers that are reused should be well-tested after each use to make sure they are still working well
Is reuse safe?
Reuse is generally considered safe when it is done properly. All dialysis centers that reuse dialyzers follow the guideline developed by the Association for the Advancement of Medical Instrumentation. These guidelines were developed with the help0 of patients, scientists, doctors and other health proffesionals, goverment officials and industry.
How is a dialyzer prepared for reuse?
Before a dialyzer can be reused, the following steps must be done:
- The dialyzer is rinsed and cleaned, either by hand or with a machine. Doing this by machine is generally safer.
- The dialyzer is tested to make sure there are no broken fibers and still working
- The dialyzer is filled with a germicide (chemical solution used to kill germs)
- When the dialyzer is ready to use, the germicide is rinsed out
- The dialyzer is tested to make sure no germcide is left, and the dialyzer can be used safely
Why do you reuse a dialyzer?
Dialyzer reuse can reduce or eliminate the physical reaction some patients have to certain dialyzer membranes. In addition, dialyzer reuse allows the dialysis center to use high-flux dialyzers, which are more costly. High-flux dialyzers are more porous and clear larger toxins from your blood.
What is reuse of a haemodialyzer?
During haemodialysis, a haemodialyzer, or artificial kidney, is used to filter fluids and wastes from a dialysis patient's blood. Reuse of a haemodialyzers means that the same haemodialyzer (filter) is used more than once for the same patient. When dialyzers are reused, they are cleaned and disinfected after each treatment. They must also be tested to make sure they are still working well before they are used again.
Management of dialyser blood leak during haemodialysis part 3
False blood leak alarm or negative to blood on multistix
- Check the blood circuit for air and remove by placing the circuit into recirculation until alarm is clear and no visible air is seen
- If the blood leak alarm cannot be reset - remove machine from service and request technical attention
- If the dialysis machine has not been internally disinfected, document on the technician request repair form
Management of dialyser blood leak during haemodialysis part 2
What happens following a blood leak alarm?
- Blood pump will stop
- Arterial and venous clamps close
- Dialysate fluid will bypass the dialyser
Why?
To ensure no neat dialysate fluid passes through the rupture and into the patient's blood
Interventions to be performed
- Check blood pump has stopped
- Clamp both arterial and venous patients access and blood circuit lines
- Check fluid in dialysate tubes for signs of discolourisation
- Non-visible blood can be checked for by removing the out-flow dialysate tube and allowing some fluid from the dialyser to moisten a urine multistix
- True blood leak - dispose of the whole blood circuit. NEVER return any blood from the circuit to the patient
- Check patients observations (blood pressure, pulse, temperature, respiratory rate, O2 saturations)
- Re-prime a full dialysis circuit again and complete the patients prescribed dialysis
- Ensure the dialysis machine completes a heat citric internal disinfection following dialysis
- Record as a dialysis incident as per local protocol
- If the dialyser problem is manufacturing fault. Notify the manufacturer with the equipment batch and lot numbers and assess whether further stock needs withdrawing from use
Management of dialyser blood leak during haemodialysis part 1
Rupture of a dialyser membrane can occur due to :
- Dialyser being dropped
- Application of excessive trans-membrane pressure during isolation ultrafiltration of excessive fluid removal
- Clotting of the blood circuit
- Clotting of the dialyse
False alarms (which activate the machine alarm system as if it were a true blood leak), can be caused by tiny air bubbles passing through the intact membrane. This is generally caused by inadequate priming before dialysis has commenced on air which has entered the blood circuit during the treatment requiring "recirculating" until all air is removed and the alarm cleared
Management of clotted circuit causing blood loss part 3
Actions if dialysis circuit is thoght to have clotted:
- Ensure access is still patent and flush with 0.9% sodium chloride for injection
- Establish reason for clotted circuit, such as inadequate anti-coagulation, over-excessive ultrafiltration, blood flow<200ml/min, rising venous pressure, dropping arterial pressure, rising trans-membrane pressure, high haemoglobin etc
- Once the clotted circuit reason/cause has been identified, re-prime a new set of blood lines and ensure no further loss of blood through clotting occurs again
- Where necessary rehydrate the patient, by infusion 0.0% sodium chloride for injection to the volume of blood loss if required
- At the patient next dialysis session, check haemoglobin if the previous results is <100 g/l and if loss is greater than 100 ml
- Complete documentation and incident report, indicating estimated blood loss volume
Management of clotted circuit causing blood loss part 2
Signs to help recognise when a circuit may be clotting are:
- Venous blood returning is much darker than blood in arterial line or pre dialyser
- Blood clots and line of blood is visible around venous chamber
- Venous pressure increases
- Transmembran pressure rises
- Air detector alarm is activated
Management of clotted circuit causing blood loss part 1
A dialysis extraporeal circuit (containing blood lines and a dialyser) contains between 200-300mls. This blood filled circuit can clot at any stage resulting in blood loss to the patient.
Measures to minimise the risk of clotting within the circuit include:
Ensuring adequate anticoagulation is used
Maintaing optimal blood flow
Removing fluid within the patients safe/tolerated limits (no more than 5% of body weight or 10-15 mls/kg/hour)
Ensuring that the dialysis machine tests are complete and passed, indicating the machine is safe to use
Management of air embolism during haemodialysis part 6
Management of an air embolism
For patient with a kidney dialysis catheter:
- Stop blood pump. Re-position the patent and flush lumens with 0.9% sodium chloride for injection
- If blood flow is achieved but still below the patient's prescribed blood flow, consider intra-dialysis infusion
Management of air embolism during haemodialysis part 5
Recognising an air embolism:
Patients complains of sudden chest pain, shortness of breath, cyanosis, seizure, cardiac/respiratory arrest
Management of an air embolism:
- Stop blood pump and clamp blood lines
- Place patient on left side with head lower than their heart (Tredelenburg position)
- Administer 100% oxygen
- Call for help and medical support if within the hospital
- Monitor and record pulse, respirations, blood pressure and oxygen saturations
Management of air embolism during haemodialysis part 4
Recognising air is detected in the dialysis machine circuit:
Visible air is detected in the dialysis circuit en-route to the patient. Venous air chamber alarm recognises air in the circuit
Management of identifying air in the dialysis machine circuit:
- Stop the blood pump
- Disconnect the arterial and venous lines from the patient and recirculate the blood lines
- Flush the patient access with 0.9% sodium chloride for injection
- Circulate the blood circuit until all evidence of air has been removed, by colecting it in the venous air chamber
- Where there is no further evidence of air in the circuit, reconnect the patient and re-commence dialysis
Management of air embolism during haemodialysis part 3
Preventing air from entering the patient and causing an air embolism:
- Ensure all connections(blood circuit, dialysis/fistula tubing to dialysis circuit, clamps, caps) are closed or capped
- Ensure dialysis catheter is not cracked and that clamps function correctly to seal the ends of the lumens
- Ensure venous dialysis circuit line is securely placed in venous air detector and line clamp function correctly
- Monitor any infusions carefully and where possible add these via venous air chamber. Packed red cell transfusions should however always be infused via the arterial port
- Where possible have patient lying/sat above the level of the blood pump
Management of air embolism during haemodialysis part 2
Air emboli may occur:
- During preparation of the dialysis catheter, if the dialysis catheter is not clamped when the end cap is removed, air may enter into the patients circulations
- If dialysis needles/access are dislodged air may enter the circulation
- Via the dialysis circuit, when the dialysis circuit lines and catheter or needle connections are not secured adequately, or the dialysis circuit is not primed thoroughly, air may enter the patient's circulation
- Once in "dialysis mode" should air enter the blood circuit, this will be detected and stopped from entering the patient via the venous air detector. However if air enters via the arterial line of dialysis blood circuit before the blood pump, it is possible for this air through gravity, to flow back to the patient be lying/sat lower than the blood pump
Management of air embolism during haemodialysis part 1
There is a risk of air embolism during the preparation of the patient's dialysis access and via the dialysis machine blood circuit. It is a serious event, with the symptoms and consequences depending on the site of passage of the air embolus within the circulation. It can lead to peripheral circulatory obstructions, convulsions, stroke or a cardiac event. The risk of air embolus is increased if the patient/nurse repeatedly silences an air alarm. during an air alarm both the venous and arterial machine line clamp close. Overriding these alarms re-opens the clamps allowing the blood flow and air to either flow backwards up the arterial line or past the venous aid chamber to the patient .
Management of nausea and vomitting during haemodialysis
Nausea and vomiting during dialysis can occur for a number of reason:
- Motion sickness from the journey to dialysis
- Food consumed just before or during dialysis. Dialysis may divert blood flow away from the gut
- Rapid fluid removal
- Hypotension
- Non-dialysis related reasons e.g. intercurrent illness
Prevention and management:
- Address any intercurrent illness, seeking medical advice if required
- Advice patient not to eat prior to and during dialysis
- If nausea is related to travel, consider prescribing anti-emetics for the patient to take prior to commencing their journey
- Commence dialysis slowly, increasing blood flow during first 30 minutes of treatment
- If related to hypotension or excessive fluid removal refer to guidelines for weight and blood pressure management on haemodialysis
Management of headache on haemodialysis part 2
Intervention:
- Check observations-treat cause if able using listed intervention
- Discuss with consultant if blood pressure markedly raised
- Administer prescribed analgesia
- Consider reducing blood flow (but not less 250 ml/min)
- Reassess fluid removal and dry weight (if considered too low)
- Consider stopping ultrafiltration until headache has settled
- Consider changing from heomodialysis to haemodiafiltration
- Treat magnesium defisiency
- Consider adjusting dialysate sodium levels
Management of headache on haemodialysis part 1
Patient often experience headaches during haemodialysis and this can be for a number of reasons. If patients continue to complain of headaches on repeated occasions, the cause may not necessarily be related to dialysis and further investigation may be required. If any patient develops a sudden and very severe headache then medical advice should be sought.
Causes of headache during dialysis:
- Disequilibrium
- Blood flow to fast
- Hypertension
- Reduction in caffein levels
- Magnesium deficiency
- Dehydration
- Dialysate sodium levels too high
- Causes unrelated to dialysis
Management of infection in haemodialysis patient part 6
Management:
- Take blood for blood cultures, C-reactive protein, urea/electrolites
- Administer paracetamol (if none taken within last 4 hours)
- Swab all catheter exit sites and wound
- If applicable obtain a mid-stream urine for assessment for urinary tract infection
- If any concerns that this is more than a simple infection then should be discussed with the unit consultant. The patient may need admission or hospital review
- Ensure antibiotics are administered appropriately before end of session
- If there are concerns that the patient is displaying signs as indicated by observation that they are seriously unwell then other measures should be instituted as appropriate
Subscribe to:
Posts (Atom)